Article Text
Abstract
Introduction Fever is one of the most common reasons for consultation in the paediatric emergency department (ED). Because of fear of bacterial infection in parents and caregivers, clinicians often overprescribe laboratory tests and empirical antibiotic treatment. The aims of this study are to demonstrate that using a procalcitonin (PCT) rapid test-based prediction rule (1) would not be inferior to usual practice in terms of morbidity and mortality (non-inferiority objective) and (2) would result in a significant reduction in antibiotic use (superiority objective).
Methods and analysis This prospective multicentric cluster-randomised study aims to include 7245 febrile children aged 6 days to 3 years with a diagnosis of fever without source in 26 participating EDs in France and Switzerland during a 24-month period. During first period, all children will receive usual care. In a second period, a point-of-care PCT-based algorithm will be used in half of the clusters. The primary endpoints collected on day 15 after ED consultation will be a composite outcome of death or intensive care unit admission for any reason, disease-specific complications, diagnosis of bacterial infection after discharge from the ED for the non-inferiority objective and proportion of children with antibiotic treatment administered for the superiority objective. The endpoints will be compared between the two groups (experimental and control) by using a mixed logistic regression model adjusted on clustering of participants within centres and period within centres.
Discussion If the algorithm is validated, a new strategy will be discussed with medical societies to safely manage fever in young children without the need for invasive procedures for microbiological testing or empirical antibiotics.
Ethics and dissemination This study was submitted to an independent ethics committee on 17 May 2018 (no. 2018-A00252-53). Results will be submitted to international peer-reviewed journals and presented at international conferences.
Trial registration number NCT03607162; Pre-results.
- procalcitonin based algorithm
- fever without source
- cluster-randomised study
- invasive bacterial infection
- procalcitonin rapid test
- paediatric emergency departements
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- procalcitonin based algorithm
- fever without source
- cluster-randomised study
- invasive bacterial infection
- procalcitonin rapid test
- paediatric emergency departements
Strengths and limitations of this study
The present study is a minimal constraint study, with very small risk of missing an invasive bacterial infection (IBI) diagnosis in practice, that was approved by referral centres and groups of experts.
The tested procalcitonin (PCT)-based algorithm (diagnostic fever (DIAFEVER)) is one of the first predictive clinical rules combining clinical and biological risk factors with a PCT rapid test to identify young febrile infants with very low risk of IBI.
If the diagnostic performance of this predictive rule is confirmed, we would be able to safely care for young febrile children at low risk of IBI without the need for invasive procedures for microbiological testing or empirical antibiotics.
Confirmation of the diagnostic performance of this predictive rule would have collective benefits by limiting the adverse outcomes during and after the antibiotics period.
The potential limitations of the study are (1) the included febrile children differing from those who consult in primary care, (2) the difficulty obtaining the consecutive and exhaustive inclusion of all young febrile children cases occurring in emergency departments, (3) during the study, clinicians being more vigilant about their practices and spontaneously decreasing the number of laboratory tests ordered and antibiotic treatments prescribed (Hawthorne effect) and (4) the included children were <3 years old. Nevertheless, the management of febrile children without source is the same at any age, following the algorithm of febrile children after age 3 months, so results may be extrapolated to the broad population of febrile children.
Introduction
Fever is one of the most common reasons for infants presenting to paediatric emergency departments (EDs). Most febrile episodes are caused by self-limiting viral infections, but bacterial infection remains a major cause of childhood mortality in industrialised countries, in which 7% to 25% febrile children1–4 have serious bacterial infections (SBIs) including urinary-tract infection (UTI) and bacterial gastroenteritis and invasive bacterial infections (IBIs) such as bacteraemia and meningitis. To avoid complications or death, physicians adopt a minimum-risk approach for children with fever without source, which can lead to overinvestigation and overtreatment resulting in unnecessary invasive testing, inappropriate antibiotics treatment prescription and unnecessary hospitalisation.
Clinical prediction rules (CPRs), by combining clinical signs, symptoms and additional diagnostic test results, could improve diagnostic decision-making for these bacterial infections.5–11 However, previously published CPRs seem to perform poorly in the ED setting, with no demonstrated impact on clinical practice and rates of discharge from the ED and few external validation studies. Highly accurate CPRs that incorporate newer laboratory tests and biomarkers such as procalcitonin (PCT)12–15 are needed to identify low-risk infants who do not require invasive diagnostic testing, empirical antibiotics treatment or admission. Looking ahead, host expression patterns such as RNA biosignatures suggest a new diagnostic paradigm,16 17 although these tools will require additional refinement and validation before their introduction to clinical practice.18 Thus, the appropriate approach to the diagnostic evaluation of febrile infants is still an area of clinical debate, and we are searching for a reliable screening test to identify young febrile infants with IBIs and to allow for safe discharge from the ED for children at very low IBI risk.19 20
In this context, in 2016, we performed a preliminary prospective study to identify young febrile infants with very low risk of IBI among 1060 young children with fever without source admitted to a university paediatric ED.21 Univariate analysis and multinomial logistic regression analysis identified independent clinical and biological risk factors of bacterial infection. PCT level seemed to be more performant than C-reactive protein level in the diagnosis of IBI in young febrile children, especially those who present to the ED with very-early-onset fever. We combined the selected clinical and biological risk factors with a newly available PCT rapid test (point of care (POC)) and built a sequential algorithm for IBI and SBI risk stratification.22–27
We hypothesise that this new POC PCT-based predictive algorithm (the diagnostic fever (DIAFEVER) algorithm) could be a highly valuable diagnostic tool to identify a group of children at very low risk of IBI and could limit unnecessary blood tests and antibiotic treatment prescriptions. This study aims to prospectively study the impact of the DIAFEVER algorithm in an open, cluster-randomised, controlled before–after clinical trial with two parallel groups in a large and multicentric cohort of febrile children <3 years old. We choose to include children <3 years old, as the previous study of Lacour et al.13 Indeed, we know that SBIs, especially IBIs, are more frequent in children with fever without source who are <3 months old. Nevertheless, there still may be severe bacterial infections in children aged 3 months to 3 years old. Severe bacterial infections become much less frequent after 3 years of age, according to the previous study conducted in 2016.21 The aims of this study will be to demonstrate that with use of the POC PCT-based DIAFEVER prediction rule (1) morbidity and mortality rates are not greater than with usual practice (non-inferiority objective) and (2) antibiotic treatment use is significantly reduced within 15 days after the first consultation (superiority objective).
Methods
Participants, interventions and outcomes
Study setting and population
This study will involve 26 investigation centres, university or general hospitals, with paediatric emergency care in France and Switzerland (figure 1, online supplementary appendix 1). Children included in the DIAFEVERCHILD study will be 6 days to 3 years old, admitted to 1 of the 26 participating EDs for an initial visit with an acute illness for a maximum of 8 days, and receiving a diagnosis of fever without source, defined as body temperature >38°C measured at home or at the ED and normal physical examination from a senior ED clinician. Children will have no current antibiotic treatment or antibiotic treatment within the 48 hours before the ED presentation. Parents will have to speak French fluently. Written informed consent will be requested from one of the parents or caregivers of the patient in case of agreement to biocollection. Only oral non-opposition will be requested otherwise.
Supplemental material
Localisation of the 26 investigation centres in France and Switzerland participating in the DIAFEVERCHILD study. DIAFEVERCHILD, diagnostic algorithm used for febrile child.
Children will be excluded if (1) a clear source of fever is identified after careful inspection of medical history and a physical examination (which means that the diagnosis is established after medical history taking and physical examination), (2) children have no fever on consultation or fever is previously subjectively assessed by parents without use of a thermometer, (3) children received ongoing antibiotic treatment or antibiotic treatment within the 48 hours before ED presentation, (4) children with fever without source revisit the ED after their initial visit, (5) parents refuse to participate or (6) children are already involved in another interventional study with human subjects or are in the exclusion period at the end of a previous study involving human subjects.
Children with fever without source who will revisit the ED after their initial visit for the same febrile episode will be included only once for this episode, and the case report form will be completed, according to the clinical course.
Intervention
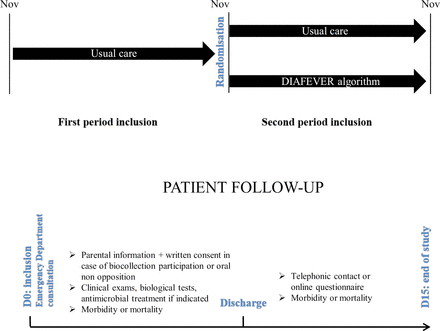
This trial will take place during 2 years and each period will last 1 year (November to October), including a whole winter in each period, as in the study timeline reported by Caille et al.28 During the first period over one winter, all centres will start with an observational period when children will receive usual care according to the usual protocol of each ED. The decision to perform any biological test, hospitalise or begin an antimicrobial treatment will be at the discretion of the physician in charge. During the second winter period, groups of children will be randomised in cluster by centres in a 1:1 ratio. The DIAFEVER algorithm will be applied in the intervention group in half of the clusters. In the remaining clusters, children will receive usual care (figure 2).
Study schedule. DIAFEVER, diagnostic fever.
The DIAFEVER clinical predictive rule consists of three age-specific algorithms (online supplementary appendix 2) and for each category, a three-level risk stratification, coloured in online supplementary appendix 2 according to the degree of risk of SBI. Corresponding recommendations for diagnostic testing, ambulatory or hospitalisation surveillance and empiric antimicrobial treatment indication are proposed as follows:
Supplemental material
With high infectious risk, coloured red in online supplementary appendix 2, the following tests and treatment should be performed:
Blood cultures.
Urinary analysis (dipstick for all infants, microscopic examinations and systematic urine culture for infants < 3 months old).
Lumbar puncture for cerebrospinal fluid culture in infants <1 month old.
Parenteral empirical antibiotic treatment for 48 hours while waiting for microbiological cultures results.
Systematic hospital admission for children <3 months old, short hospitalisation or ambulatory management considering clinical symptoms and familial possibility for surveillance in children >3 months old.
With intermediary infectious risk, coloured orange in online supplementary appendix 2, the following tests should be performed:
Blood cultures.
Urinary analysis (dipstick for all infants, microscopy examinations and systematic urine culture for infants <3 months old).
Hospital surveillance for about 1–3 hours, except for children <1 month old, who will always be admitted.
With low infectious risk, coloured green in online supplementary appendix 2:
Discharge.
Symptomatic fever treatment.
Oral and written surveillance information given (online supplementary appendix 3).
Re-consultation in case of worrying symptoms or persistent fever >48 hours.
Supplemental material
Toxic symptoms or signs are as follows: irritability, lethargy, low capillary refill, tachypnoea, cyanosis, chills, marbling, bulging fontanelle, serious concerns expressed by the parent(s) or the clinician, a temperature ≥40°C and purpura at home or at the PED.
During the use of the DIAFEVER algorithm, dipstick testing should be performed with urine obtained by clean catch or <20 min after emission with bag catch. Positivity for leucocyturia will be considered with a trace visible on the urinary dipstick or with nitrite detection with or without associated leucocyturia. Urinary culture will be indicated with a positive dipstick result, but culture should be performed with urine collected by an aseptic technique only: bladder catheterisation or spontaneous urine emission after cleaning.
For determination of the PCT value, we will use the B.R.A.H.M.S PCT-direct system (ThermoFisher), which has a measurement range of 0.1–10.0 µg/L, requires 20 µL capillary blood obtained by finger prick with a standard protocol26 and provides a result in 20 min. Two weeks before the experimental period, we will train all emergency teams to perform the PCT-direct test according to the manufacturer’s instructions.
Patient and public involvement
We involved paediatric and general practitioners to write and illustrate the instructions concerning ambulatory monitoring, which will be given to the parents at the end of the visit in the ED to check their febrile child (online supplementary appendix 3). Before the beginning of the study, we also involved parents visiting in one French ED by giving them these instructions and asking about improvements after they read them.
Modifications
Any children could stop their involvement in the study because of a parental or medical decision. No adverse events related to the research are expected because of the low risks and constraints of this protocol. Therefore, the occurrence of an adverse event relating to the care of the patient during this protocol will result in a declaration with the proper vigilance system of each ED (pharmacovigilance, biomonitoring, blood safety or medical device). The research ethics committee that approved the study did not require an independent safety monitoring committee.
Adherence
To facilitate the study feasibility, a multifaceted strategy will be considered. Before each inclusion period, all team participants will be informed and trained in the study objective, design and implementation of the study. The research individuals recruited for this study will systematically check for eligible children possibly missed each day. A description detailing the rationale and conditions of this study will be placed in the ED.
Concomitant care and interventions
In the ED teams in which the PCT test is usually used in current practice, this test can be still used in the control group only if performed in the biochemical laboratory and not with a POC organisation. The decision to perform any test other than those provided for in the protocol will be at the discretion of the physician in charge. The patients will be admitted and/or receive antibiotic treatment according to the management protocol of each centre in the control period only. However, physicians are able to overrule the recommendation according to their own judgement in the DIAFEVER algorithm period.
Primary outcomes
The primary objective is to demonstrate that with use of the POC PCT-based DIAFEVER prediction rule (1) morbidity and mortality rates are not greater than with usual practice (non-inferiority objective) and (2) antibiotic treatment use is significantly reduced within 15 days after the first consultation (superior objective). The following primary endpoints will be considered:
A composite outcome considering occurrence during the 15 days after discharge from the ED of one of the following events: death, intensive care unit admission, disease-specific complications (ie, cerebral damage with neurological impairment, deafness, blindness, amputation, cutaneous necrosis requiring surgery reparation, definitive renal failure) with a diagnosis of IBI or SBI.
The proportion of children who received antibiotic treatment.
According to the previous study performed in 2016 in France,21 antibiotics exposure was 34%. Using the DIAFEVER algorithm, we would reduce the antibiotic exposure to 24%, corresponding to 10% absolute decrease and 28% relative decrease in this previous study.
An IBI is defined by the isolation of a bacterial pathogen in blood or cerebrospinal fluid culture. Staphylococcus epidermidis, Propionibacterium acnes, Streptococcus viridans, Diphtheroides, Candida albicans or other Candida species in urine will be considered contaminants. An SBI is a serious bacterial infection, including UTI (urine culture with growth of ≥10 000 cfu/mL with associated leucocyturia >10/mm3), bacterial gastroenteritis and IBI. If a registered patient has a high suspicion of bacterial infection but no positive bacterial culture, the case will be discussed among the principal investigators to decide the most appropriate classification. With positive urine culture without leucocyturia, UTI will be considered. With two different bacteria identified, the urine sample will be considered contaminated and not available for analysis.
Because the diagnosis of SBI is established during the first 48 hours after the ED consultation in 95% of cases,29 most infected children will be identified during their stay in the paediatric hospital unit. A telephone call to parents 15 days after admission in the ED will identify the remaining 5% and children will be classified according to clinical and biological course. The investigator’s research teams in each centre will have procedures to identify the children included in the study who are admitted to the intensive care unit, neonatology unit, conventional hospitalisation unit or back to the ED. A systematic weekly check between the research teams and clinical units will be organised to ensure the detection of any serious adverse outcomes in included children. In case of hospital readmission, a hospitalisation report will be collected. The classification used for all febrile children will be the perform consensus of Professor Mike Levin tested in 11 countries in March 2018.
Secondary outcomes
The secondary objectives and endpoints are as follows:
To describe the current epidemiology of fever without source with the incidence of fever without source among children admitted to EDs and the incidence of SBI and IBI among children admitted to the ED with fever without source.
To determine the diagnostic value of the DIAFEVER prediction rule for SBI and IBI diagnoses when calculating the sensitivity, specificity, predictive values and likelihood ratio of the DIAFEVER prediction rule, considering the SBI and IBI diagnosis as the gold standard.
To determine the impact of the DIAFEVER prediction rule on ED care organisation by reporting the median length of stay in the ED, the prescription rate of laboratory tests and hospitalisation rates.
To determine vaccine coverage of children consulting for fever without source and theoretically vaccine-preventable morbidity and mortality of SBI by using the vaccination coverage rate and theoretically vaccine-preventable SBI, defined as an infection with an identified serotype included in the national vaccine schedule and occurring in a child with untimely vaccination.
Ancillary
An ancillary study consisting of the biocollection is planned to perform complementary studies in the field of the transcriptomic biosignature and to obtain further results on the genetic tests recently reported in febrile adults and in children.16 30 31 The aim of this biocollection was to identify proteomics, transcriptomics and genomics biomarkers to better stratify the infectious risk of febrile children. This biocollection will be proposed to parents when some blood tests are indicated in the usual diagnostic course for their febrile child. This biocollection will be explained by a specific notification and will need a specific written consent. A supplementary blood sample (1–2 mL depending on the child’s size) will be stored at −80°C and the clinical and microbiological data will be collected in the usual DIAFEVER electronic case report form (e-CRF). The extra sample obtained for this research will be added to the biocollection titled ‘Pediatrie’, integrating the information consent form. Before the final centralisation, all biocollection samples will be stored in each centre. This biocollection and consent procedure were registered under no. ‘DC-2011–1399’.
Participant timeline
Children’s participation will last 15 days after ED consultation. The parents or caregivers of children cared for as outpatients will be asked to complete a self-reporting e-CRF online at day 15. If they do not, they will receive a follow-up telephone call within 1 month after the initial visit at the paediatric ED to check on the course of the episode. If after three telephone calls, caregivers cannot be contacted, the electronic registries of the paediatric ED and the Public Health System will be used to identify and review any following visit to the primary care centre or any other hospitals in the district area (table 1).
Participant timeline
Sample size
Between the two primary outcomes, the safety outcome was most subject-consuming outcome and therefore it was chosen to ensure the power required for the two outcomes. Assuming an incidence of fever of 1% for the entire ED population, assigned to each group, and considering a 1% non-inferiority margin, an individually randomised trial would require 3120 patients to achieve 80% power. Taking into account clustering, considering that we will have 26 centres and that the intraclass correlation coefficient will be 0.001, the total required sample size is 7200. We planned to perform both an intention-to-treat and a per-protocol analysis and expect 4% of children to be excluded for the per-protocol analysis. Therefore, we plan to recruit 7245 patients.32
Recruitment
If 2.5% of the patients admitted in paediatric EDs have the inclusion criteria,21 collectively, the 26 participating centres receive approximately 23 000 febrile young children/year. Given a recruitment coverage of 40% (children would be included especially during the day, when health caregivers’ number is higher) and assuming 40% loss due to ED overcrowding and forgotten inclusion by overworked teams, 10% parental opposition and 10% lost inclusion at day 15; the potential recruitment estimate is 9200 young febrile children per year. This very secure calculation will lead to a total of 9200 patients included although only 7245 patients are needed for this project. Thus, the feasibility of the recruitment is ensured during a 24-month period.
To assure consecutive enrolment, many doctors in each ED will be involved in the study. They will ensure that their team (students, young doctors and seniors) will enrol patients consecutively. Moreover, clinical research members of each ED will check, during the day, reasons for admission of each child and will write a note on the emergency board away from the ED, in case of the children could be enrolled in the study after clinical examination.
Assignment of interventions
Allocation
All centres will be allocated to a treatment: control allocation ratio 1:1. The statistician will perform the randomisation by using a computer program written in R V.3.2.0. Incrementation will be randomised by the 1415 INSERM team (Tours Hospital) by BG and ET. The senior ED clinician, after checking inclusion criteria and performing a physical examination, will enrol children in the study and assign them to the DIAFEVERCHILD algorithm, according to the cluster.
Blinding
The assessed intervention does not allow physicians to be blinded. Parents will not be informed as to whether the care was via the DIAFEVER algorithm or usual care. Parents who do not complete the e-CRF at the follow-up will be contacted by phone. Interviewers will be asked to be very careful to not inform parents about the group to which their children have been allocated. Interviewers will not be blinded but statisticians will be blinded.
Data collection and management
Data collection
The following data are recorded: demographics, medical history, birth context for children <3 months old, vaccination schedule, temperature registered at home and at the PED, home fever management, time between when fever was first detected and when the infant was brought to hospital, date and time of admission and exit of ED, clinical examination at the admission, parental and medical concerns for the child, results of any tests performed, treatment received, diagnosis and orientation after the visit in the ED (return home or hospitalisation).
The following additional data on day 15 were also collected by phone call to parents: clinical pathway, tests performed or treatments received in the meantime, child’s condition now, utility of instructions for fever (online supplementary appendix 3) given at the end of the visit in the ED.
Electronic case report form
Data for each patient participating in the research will be collected by an e-CRF developed by using Ennov Clinical. All information required by the protocol will be provided in the e-CRF, including data required to confirm compliance with the protocol and all data necessary for statistical analysis and to identify major deviations from the protocol. Entering, viewing or modifying data will only be possible via the e-CRF pages (input masks), at https://nantes-lrsy.hugo-online.fr/CSonline. The data will be stored directly from the e-CRF into the database hosted on a dedicated server, with controlled access. Any addition, modification or deletion of data will be recorded in a non-editable electronic file. The e-PRO module of Ennov Clinical will be used to managed the parent interview on day 15. Parents or legal guardians can electronically complete the online questionnaire on day 15 at https://nantes-lrsy.hugo-online.fr/CSePro. Their access is separate from that of the investigators. The clinical team can contact and relaunch the patient via the system’s email. For that purpose, the parents or legal guardians will have to give their email address.
Statistical methods
Statistical analysis of the primary outcome
The primary endpoints will be compared between the experimental and control groups by using a mixed logistic regression model adjusted on clustering of participants within centres and period within centres.
To demonstrate non-inferiority on the composite primary outcome, the two-sided 95% CIs of ORs must be below the predefined non-inferiority margin. The non-inferiority margin initially defined considering a difference in proportion will be transformed in that it translates to a non-inferiority margin for an OR considering the following equation: OR=[(1− ) (δ+
) (δ+ )]/[
)]/[ (1−
(1−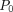 −δ)], where
−δ)], where 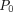 is the incidence rate in the control group and δ the difference in proportion. Thus, a non-inferiority margin a priori-specified as 1% and an expected a priori-specified incidence rate of 1% in the control group leads to a non-inferiority margin for the OR of 2.02. If the margin is included in the 95% CI, the result will be inconclusive. Both per-protocol and intention-to-treat analyses will be performed. Differing results from the per-protocol and intention-to-treat analyses will be defined as inconsistent.33
is the incidence rate in the control group and δ the difference in proportion. Thus, a non-inferiority margin a priori-specified as 1% and an expected a priori-specified incidence rate of 1% in the control group leads to a non-inferiority margin for the OR of 2.02. If the margin is included in the 95% CI, the result will be inconclusive. Both per-protocol and intention-to-treat analyses will be performed. Differing results from the per-protocol and intention-to-treat analyses will be defined as inconsistent.33
Statistical analysis of secondary outcomes
The incidence of fever without source in ED consultations will be estimated by pooling data for the two groups: experimental and control.
The incidence of SBI and IBI in children consulting for fever without source will be analysed in the framework of a mixed logistic regression. Associated clinical symptoms, condition evolution, microbiological culture documentation and antibiotic resistance among isolated bacteria will be reported by using descriptive statistics. The diagnostic properties of the DIAFEVER prediction rule will be assessed considering only patients who will be recruited in centres applying the rule and during the ad hoc period. Point estimates and associated 95% CIs of the different indexes will be estimated.
The median length of stay in the ED, reported in hours, will be analysed in the framework of a mixed linear regression model. The proportion of children with laboratory tests will be compared in the framework of a mixed logistic regression model. The number of laboratory tests per children will be compared in the framework of a mixed Poisson regression model. The hospitalisation rate will be analysed in the framework of a mixed logistic regression model.
The vaccination coverage rate and the theoretically vaccine-preventable SBI rate will be estimated by pooling the two groups: experimental and control.
Missing data
Missing data will be handled by simple imputation considering that no morbidity and mortality event occurred and no antibiotic treatment was prescribed.
Monitoring
Data monitoring
Data monitoring will be performed by the research division promotion department. A clinical research associate will visit each site regularly to conduct quality control on the data reported in the case report forms. The members performing this function are not yet known. The on-site monitoring visits will be organised after making arrangements with the investigator. The clinical research associate should be able to consult the following on each site: the enrolled patients’ data compilation records, the patients’ medical and nursing files, study-related charts and the investigator file. No interim analysis is planned because the protocol of the study has been classified according to the estimated level of risk for the patient as low or negligible foreseeable risk (risk A).
Harms
No adverse events related to research are expected because of the low risks and constraints of this protocol. Therefore, the occurrence of an adverse event relating to the care of the patient during this protocol will result in a declaration to a proper vigilance system.
Auditing
Within the scope of this study, an inspection or audit may be conducted. The sponsor and/or participating centres should be able to provide inspectors or auditors with access to the data.
Ethics and dissemination
Research ethics approval
The data gathered during the study will be held in a computerised file, as per the 2004 amendment of the French data protection act of 6 January 1978. The protocol falls within the scope of the MR001 methodology applied by Nantes University Hospital. This study was submitted to an independent ethics committee on 17 May 2018 (no. 2018-A00252-53). The study was also submitted to the relevant Swiss Ethical Review Board and competent authorities for prior approval to include patients in Switzerland.
Protocol amendments
Requests for substantial modifications to the protocol will be reported to a National Security Medicine Agency and for approval and notification to the Ethical Review Board concerned in compliance with the law and its implementing decrees. The patient information and consent forms will be amended if required.
Consent or assent
After verification of inclusion criteria, the investigator will inform parents or legal guardians about the protocol with clear and precise information and request from one of parent or legal guardian, at least a written and signed consent form in case of requests for biocollection only (online supplementary appendix 4). If no biocollection sample is performed, oral non-opposition will be requested from one of the parents.
Supplemental material
Confidentiality
Each patient’s medical data will be provided only to the sponsor (research department of Nantes University Hospital) or any person duly authorised by the sponsor, and, when applicable, to authorised health authorities, under confidential conditions. The data compiled during the trial will be processed electronically in compliance with the French Data Protection Authority for clinical research requirements.
Access to data
The data management coordinating centre, Nantes University Hospital (CGL and EL), will oversee the intra-study data-sharing process, with input from the Data Management Subcommittee. All datasets will be password-protected. Project principal investigators will have direct access to their own site’s datasets and will have access to other site data on request. To ensure confidentiality, data disseminated to project team members will involve blinding of any identifying participant information.
Dissemination policy
Results will be submitted to international peer-reviewed journals. Authorship will be defined according to ICMJE; no professional writer will be involved. A copy of the publication will be delivered to the sponsor (University Hospital of Nantes) and given to caregivers of included children if requested. Results will also be presented at paediatric national and international conferences. All topics suggested for presentation or publication will be circulated to the main investigator, CGL, at University Nantes Hospital.
If the project demonstrates the impact of the DIAFEVER algorithm on morbidity–mortality and reduction of antibiotic treatment use, a new strategy related the management of febrile children could be discussed with medical societies to standardise practices.
References
Footnotes
Contributors GH and CFF initiated the study design, designed the data collection instrument and drafted the initial manuscript. EL initiated the study design, designed the data collection instrument, drafted the initial manuscript, helped with implementation and provided methodological and statistical expertise in clinical trial design. AC-D, FL, ET and BG helped with implementation and provided methodological and statistical expertise in clinical trial design. CGLG conceptualised the study, initiated the study design, designed the data collection instrument and drafted the initial manuscript. All authors contributed to refinement of the study protocol and approved the final manuscript. Principal investigator and research physician (CGLG): design and conduct of DIAFEVER algorithm, prepare protocol and revisions, prepare investigators brochure and CRFs, organise steering committee meetings, manage Clinical Trials Office and publish study reports. Steering committee: agrees with final protocol, recruits patients, liaises with principle investigator and reviews progress of study. Trial Management Committee (CGLG, EL, ET, BG): study planning, organise steering committee meetings, provide information on serious unexpected suspected adverse events, responsible for trial master file, give advice for lead investigators, check data, randomise and organise serum sample collection. Lead investigators: in each participating centre, a lead investigator is identified to be responsible for identification, recruitment, data collection and completion of case report forms, along with follow-up of study patients and adherence to study protocol and investigator brochure.
Funding The DIAFEVERCHILD study received a French national grant from the French Ministry of Health (PHRC no. 17-17-0354). ThermoFisher will provide instruments to analyse PCT rapid tests.
Map disclaimer The depiction of boundaries on the map(s) in this article do not imply the expression of any opinion whatsoever on the part of BMJ (or any member of its group) concerning the legal status of any country, territory, jurisdiction or area or of its authorities. The map(s) are provided without any warranty of any kind, either express or implied.
Competing interests The DIAFEVERCHILD study received a French national grant from the French Ministry of Health. ThermoFisher will provide instruments and tests for the micromethod PCT assay, the B.R.A.H.M.S PCT-direct system.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication Not required.
Provenance and peer review Not commissioned; externally peer reviewed.