Article Text
Abstract
Introduction Individuals with Parkinson’s disease (PD) often experience initial hesitation, slowness of movements, decreased balance and impaired standing ability, which can significantly impact their independence. Transcranial magnetic stimulation and transcranial direct current stimulation are two widely used and promising non-invasive brain stimulation (NIBS) modalities for treating PD. The supplementary motor area (SMA), associated with motor behaviour and processing, has received increasing attention as a potential stimulation target to alleviate PD-related symptoms. However, the data on NIBS over SMA in PD individuals are inconsistent and has not been synthesised. In this article, we will review the evidence for NIBS over SMA in PD individuals and evaluate its efficacy in improving PD function.
Method and analysis Randomised controlled clinical trials comparing the effects of NIBS and sham stimulation on motor function, activities of daily living and participation for people with PD will be included. A detailed computer-aided search of the literature will be performed from inception to February 2023 in the following databases: PubMed, EMBASE, Physiotherapy Evidence Database (PEDro), Web of Science (WOS) and The Chinese National Knowledge Infrastructure (CNKI). Two independent reviewers will screen articles for relevance and methodological validity. The PEDro scale will be used to evaluate the risk of bias of selected studies. Data from included studies will be extracted by two independent reviewers through a customised, preset data extraction sheet.
Ethics and dissemination Ethical approval is not required for this systematic review. The study’s findings will be presented at scientific meetings and published in peer-reviewed journals.
PROSPERO registration number CRD42023399945.
- non-invasive brain stimulation
- Parkinson-s disease
- supplementary motor area
- protocol
- meta-analysis
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
To the best of our knowledge, this will be the first review of the literature examining non-invasive brain stimulation (NIBS) over supplementary motor area (SMA) interventions specific for recovery Parkinson’s disease.
The overall effects of NIBS over SMA will be estimated by meta-analysing outcomes data and the quality of the body of literature will be evaluated using Grading of Recommendation, Assessment, Development and Evaluation.
Studies designed as cross-over experiments will be included, which may have residual effects.
Subgroup and sensitivity analyses will be used to explore potential heterogeneity.
Restriction of publication language to English only is a limitation of this study.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder that causes progressive motor symptoms. Approximately 6.1 million people suffered from PD worldwide in 2016, which is projected to increase to over 12 million worldwide by 2050.1 Motor symptoms of PD include bradykinesia, rigidity, rest tremor, freezing of gait (FOG), postural instability and balance dysfunction. Some symptoms are present in early PD and increase with disease progression, which may lead to an increased risk of falls, limited social participation and impact their quality of life.2–4 Sixty-eight per cent of patients with PD sustain at least one fall per year which is double the fall rate reported in healthy older adults.5 Pharmacological treatments, such as levodopa therapy, were commonly used to relieve motor symptoms. However, more than 40% of individuals treated with oral dopamine agonists experience impulse control disorders.6
The two most common types of non-invasive brain stimulation (NIBS) for treating PD are transcranial direct current stimulation (tDCS) and repetitive transcranial magnetic stimulation (rTMS).7 8 rTMS is a non-invasive technique that uses stimulation coils placed on the scalp to deliver repeated magnetic pulses to specific areas of the brain. This depolarises neurons and generates activity at synaptic terminals, modulating physiological brain function. rTMS is a relatively safe therapeutic approach for PD with few significant adverse effects, such as seizures, hearing impairment or mania. If appropriately evaluated and supervised, rTMS can be used securely on a substantial percentage of sufferers.9 10 rTMS at high frequency (≥5 Hz) can enhance motor cortex excitability, whereas low-frequency rTMS (≤1 Hz) can depress cortical excitability. Chou et al demonstrated the efficacy of rTMS with a moderate effect size for patients with PD.11 A meta-analysis has shown HF-rTMS on bilateral M1 region is effective for global PD motor performance.12 tDCS delivers a continuous current that modulates membrane excitability and induces shifts in cortical excitability, with the polarity defining the effects. Several studies have shown that tDCS is safe and effective in the treatment of a variety of neurological diseases.10 13 Pol et al14 demonstrated that anodal tDCS over the motor area is a promising intervention approach for the improvement of gait in PD. There are numerous choices of stimulation targets for NIBS, including the primary motor cortex (M1), the dorsolateral prefrontal cortex15 and the cerebellum.16
Recently, researchers paid more attention to supplementary motor area (SMA) as the stimulation target to improve motor functions in patients with PD. Anatomically, SMA is located in the medial part of the premotor cortex and is primarily associated with the generation of movement-related processing,17 speech18 and articulation.19 SMA is a pivotal area in the basal ganglia-cortical loop and impaired in PD.20 The signal change of SMA is directly related to disease severity, indicating the presence of SMA impairments in the early stages of PD.21 One study explored rTMS effects when applied to the SMA vs motor cortex, significant improvements in FOG were observed after SMA, but not after motor cortex stimulation,22 suggesting that SMA may be a potential target for alleviating PD symptoms. Koch et al23 found that rTMS over SMA can modulate abnormal involuntary movements in patients with PD. Meanwhile, Ma et al24 showed that high-frequency rTMS over the SMA cannot alleviate the sequence effect in PD patients with FOG.
Several systematic reviews of NIBS for PD in recent years have compared the effects of rTMS and tDCS on motor function in PD, HFrTMS may be a stimulation modality that improves motor function in patients with PD compared with tDCS, and NIBS over SMA has been proposed as a potential stimulation modality to improve motor function in PD.11 14 25 26 However, the efficacy of this stimulation modality on overall motor function and walking function in PD remains inconsistent.24 27 The efficacy of NIBS over SMA has come into confusing results, possibly due to methodological heterogeneity in the therapeutic regimen. Furthermore, while NIBS exists, none have a specific focus on NIBS over SMA in people with PD, largely because, up until recently, there has not been enough literature on the topic area. Emerging findings from various research indicate the necessity of conducting a meta-analysis to reach a more accurate estimation of the effects of NIBS over SMA in PD.
Objective
In this paper, we report on the protocol of a systematic review and meta-analysis that will:
Describe NIBS over SMA interventions for individuals with PD.
Quantitatively assess the effect of SMA over NIBS programmes on clinical outcomes in the areas of general motor function and walking activity.
Methods
This protocol will be prepared in accordance with the guidelines provided by the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) (online supplemental file 1) guidelines28 and has been registered with the International Prospective Register of Systematic Reviews (PROSPERO; registration number CRD42023399945).
Supplemental material
Criteria for selecting studies for this review
Type of studies
Randomised controlled trials (RCTs) comparing the group undergoing treatment with NIBS over SMA and the control group (sham stimulation) will be considered eligible for this study. Observational or descriptive study designs, including cohort studies, case series, case reports and non-randomised studies will be excluded.
Types of participants
Studies involving individuals with a diagnosis of PDat Hoehn-Yahr stages 2–4 who exhibited motor impairment will be included. There will be no restrictions placed on the patient’s age or gender. Studies that individuals with PD have contraindications to NIBS or with other diseases, such as Alzheimer’s disease, stroke or multiple sclerosis, will be excluded.
Types of interventions
The review will include studies that involve the use of NIBS over SMA to promote recovery of PD symptoms. For the purposes of this review, NIBS will be defined as the treatment to modify or modulate the cortical excitability29 and synaptic connectivity30 in the cerebral cortex, such as long-term potentiation and long-term depression, which are considered relevant mechanism of plastic reorganisation.31 The two most common NIBS techniques are TMS and transcranial electrical stimulation (tES).
TMS employs rapidly changing magnetic fields to induce a brief pulse of electric current on the cortex, thereby generating action potentials with a depth of up to 5 cm.32 33 TMS can be used in different ways, rTMS, intermittent theta burst stimulation (iTBS) and continuous TBS (cTBS). High-frequency rTMS (≥5 Hz), iTBS and paired-pulse rTMS (inter-stimulus interval (ISI) 1.5 ms) are considered to have facilitatory effects.32 34 In contrast low-frequency rTMS (≤1 Hz), cTBS and paired-pulse rTMS (ISI 3 ms) are expected to induce inhibitory effects.32 34 Paired-pulse TMS consists of two successive pulses, employed in research investigating the impact of TMS on neurological conditions and diseases.34 Depending on the intensity of conditioning stimulus (CS) and the test stimulus (TS) and ISI used, paired-pulse TMS is used to explore inhibitory or excitatory intracortical networks.35 The short ISI (1–5 ms) with subthreshold CS and suprathreshold TS reaching 1.5 mV motor evoked potential (MEP) will produce short intracortical inhibition.36 Reis et al37 observed that increasing MEP amplitude with the ISI of 10–15 ms will produce a phenomenon known as intracortical facilitation. Valls-Solé et al38 found that when CS intensity exceeds 110% of resting motor threshold and the ISI ranges from 60 to 150 ms, it will induce long intracortical inhibition.
tES can be further classified into tDCS and alternating Current Stimulation (tACS) depending on the type of electric current used.39 tDCS and tACS possess anodal and cathodal polarity, resulting in the hyperpolarisation or depolarisation of the resting membrane potential, respectively.40 41 The available data indicate that a direct current of at least 0.6 mA that is applied for at least 3 min is sufficient to modulate cortical excitability.
Consequently, this study will include research using NIBS over SMA as the primary intervention for individuals with PDWe will exclude RCTs that did not use NIBS techniques. If a study employs additional therapeutic interventions such as conventional physical therapy or pharmacotherapy, it will also be included.
Search strategy for identification of studies
Electronic searches
The search will be conducted across five databases, including MEDLINE, EMBASE, Web of Science (WOS), Physiotherapy Evidence Database (PEDro) and China National Knowledge Infrastructure (CNKI). No limitations will be imposed on the language or publication location. Where necessary, articles will be translated into English by an independent interpreter for analysis. Relevant literature will be identified using a combination of keywords and Medical Subject Headings (MeSH) related to TMS, tDCS, SMA and PD. Search strategies (online supplemental file 2) will be customised for each database to enhance search accuracy.
Supplemental material
Search of other sources
We will perform a handsearch of the reference lists of the studies included in the review to identify any potentially relevant studies not retrieved during the electronic search. The grey literature will not be searched.
Types of outcome measures
Studies that report results related to general motor function and walking performance using standardised or non-standardised assessments before and after the intervention or follow-up will be included. We will define the general motor function as the primary outcome and the walking ability as the secondary outcome. To investigate the effect of NIBS on general motor function in individuals with PD, we will include the motor examination section of the Unified Parkinson’s Disease Rating Scale-III and the Hoehn-Yahr stage. Additionally, we will include the Freezing of Gait Questionnaire, Timed Up and Go test, gait velocity, walking cadence and ambulation time to analyse the effect of walking ability of individuals with PD after NIBS over SMA.
Data collection and management
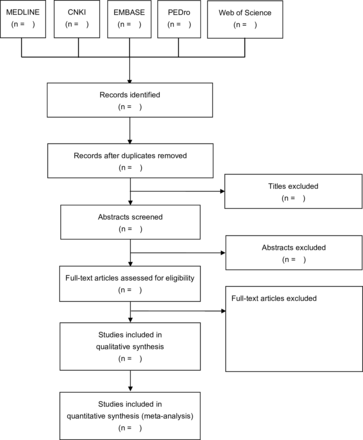
We follow the PRISMA guidelines in the study selection (figure 1). Databases will be searched by two reviewers (YW and YC) to identify potential titles and abstracts. Search results will be imported to EndNote V.20 citation software for automatic duplicate removal. Any duplicates ignored by the software will be manually removed. According to the inclusion and exclusion criteria, two independent reviewers (YW and YC) will scan the titles and abstracts of articles for screening the unrelated reports. Potentially relevant studies will then undergo full-text analysis. The entire selection process will be performed by consensus. If any disagreements can be reached on a given study, a third reviewer (SY) will arbitrate. Corresponding authors will be contacted via email for original data where the published data were insufficient for data analysis.
Flow chart of data collection and management.
Data extraction
After the selection of the studies, the two reviewers (YW and YC) will work independently. The following data will be extracted from each study: authors, country, study design, sample size, sex of participants, mean stage of PD, mean disease duration characteristics of participants; type and parameter of NIBS, intervention and control details, number of sessions and frequency of treatment, outcome measures. Disagreements about data extraction will be checked by the original text, and a final consensus regarding any inconsistency will be reached through discussion with a third reviewer (SY).
Assessment of risk of bias
Two independent reviewers (YW and YC) will use the Physiotherapy Evidence Database (PEDro) scale to assess the risk of bias.42 The PEDro scale scored 11 items as either present or absent. The final score is 10, as item 1 is not included in the score calculation as it represents external validity. We will consider a PEDro score of 11 to represent an excellent-quality study, scores of 8–10 for a good-quality study, scores of 6 and 7 for a fair-quality study, and scores of 5 or lower for a low-quality study.43 Any disagreements between reviewers will be resolved through discussion. If a consensus cannot be reached, a third reviewer (SY) will be consulted.
If sufficient data are included, we will assess reporting bias by using a funnel plot.44 A funnel plot is a graphical representation of the estimated treatment effects (x-axis) from individual studies plotted against a measure of sample size (y-axis). In the absence of bias, the results from studies with smaller sample sizes would be widely dispersed at the bottom of the graph, with the dispersion decreasing among studies with larger sample sizes. Asymmetry in funnel plots may indicate the presence of publication bias and can be used to investigate small study effects.45
Quality of evidence
Some studies may use NIBS as part of the intervention. This may limit the examination of the effect of using NIBS over SMA only on the reported outcomes. Therefore, we will use the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system46 47 to assess the strength of the evidence for each outcome. The GRADE domains include the risk of bias, imprecision, inconsistency, indirectness and publication bias for the purpose of downgrading the quality of evidence.48 Conversely, the quality of evidence could be upgraded49 in the presence of a large magnitude of effect, a dose-response gradient and any residual confounding effects that would diminish the observed effect. Two reviewers (YW and YC) will independently rate the quality of outcomes using the GRADE system.
Measures of treatment effect
For studies with comparable outcome measures, data will be pooled for meta-analysis. Continuous data will be presented as 95% CI and mean difference (MD) and confirm whether the mean effect size was significant. Significance was set at p<0.05 for all statistical analyses. The mean effect was indicated as MD with 95% CI. For dichotomous data, the risk ratio and 95 % CI will be calculated.
Dealing with missing data
If data are missing, the authors will be contacted for additional information. Should the authors be unable to provide the necessary information within a 2-month period, an intention-to-treat analysis will be applied to the extrapolated data. The implications of this approach will be discussed in the systematic review’s discussion section.
Assessment of heterogeneity
We will use Review Manager (V.5.3, Nordic Cochrane Centre, Copenhagen, Denmark) in our systematic review and meta-analysis. Summary statistics will be calculated using either the standardised MD or weighted MD with 95% CI. Heterogeneity is the true difference in effect sizes related to intrinsic factors of the studies included in the meta-analysis. If all studies share the same common effect, the fixed-effects model will be recommended; otherwise, if heterogeneity is expected, a random-effects model will be preferred.50 51 As there were several clinical differences in NIBS over SMA in PD research in terms of various aspects, including study designs, stimulation modes and frequencies, and medication doses used. Accordingly, a random effects model will be used for the meta-analysis to account for the expected heterogeneity. Qualitative descriptions will be provided if quantitative evaluation is not feasible. Statistical significance will be set at p<0.05.
Sensitivity analysis
We will conduct a sensitivity analysis by excluding studies characterized by poor quality, absence of blinded outcome evaluation, and unreported follow-up, aiming to assess the robustness of the findings.
Analysis of subgroups or subsets
After conducting initial meta-analyses, we will employ subgroup analysis to explore the impact of methodological disparities and participant heterogeneity based on various potential factors, such as distinct types of NIBS, the different effects of NIBS (facilitatory and inhibitory stimulation), and the mediation status of the people with PD (ON medication and OFF medication) during the assessment.
Patient and public involvement
There will be no direct patient or public involvement in this study, as it is a secondary study based on other studies.
Ethics and dissemination
Ethical approval is not required for this review.The study’s findings will be presented at scientific meetings and published in peer-reviewed journals. All study-related publications and presentations will be authorised and reviewed by the study investigators.
Review status
The reviewers have commenced searching relevant studies on the electronic databases. This review is expected to be complete by August 2023.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
YW and SY contributed equally.
Contributors YW, SY, H-HJ and YC were each involved in the conception, design, writing and editing of the study protocol. YQ, LZ and RM contributed to the critical revision of the manuscript for important intellectual content and approved the final version of the manuscript. All authors have read and approved the final manuscript.
Funding This study is funded by the NSFC 82172540 from the National Natural Science Foundation of China.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.