Article Text
Abstract
Introduction Awake tracheal intubation (ATI) involves placing a tracheal tube in an awake, spontaneously breathing patient. Superior laryngeal nerve block (SLNB) can effectively abolish the glottic closure reflex, and blunt the sensation of the structures above the cords. A method that consists of SLNB along with translaryngeal injection (TLI) can provide satisfactory anaesthesia and intubating conditions. We present a novel modified access of SLNB, ultrasound (US)-guided anterior SLNB, to aid awake videolaryngoscopes-assisted endotracheal intubation in adult elective surgery patients, and we compare the effectiveness and safety to traditional US-guided posterior SLNB.
Methods and analysis A total of 100 adult elective surgery patients requiring general endotracheal anaesthesia will be randomly assigned to the modified group (modified US-guided anterior SLNB) or the traditional group (traditional US-guided posterior SLNB). After SLNB, all participants will be performed with TLI. The primary outcome is the proportion of acceptable intubation conditions based on intubation scores. Secondary outcomes include: (a) the first-attempt intubation success rate, (b) haemodynamic changes during ATI, (c) time taken for airway anaesthesia and intubation, (d) recall of intubation, (e) participant perception of comfort during intubation, (f) perioperative complication rate. This report describes the study design of this randomised controlled trial.
Ethics and dissemination The study protocol has been approved by an ethical committee of the West China Hospital (Sichuan University), and registered at the Chinese Clinical Trials Register (www.chictr.org.cn). Results will be published in a peer-reviewed journal.
Trial registration number ChiCTR2200058086.
- adult anaesthesia
- ultrasonography
- pain management
- adult intensive & critical care
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This is the first randomised controlled trial designed to assess the effectiveness and safety of modified ultrasound-guided anterior superior laryngeal nerve block (SLNB) during awake tracheal intubation (we presented a novel method of SLNB which is not previously reported by others).
Appropriate non-inferiority margin and sample size calculation.
The primary outcome, the proportion of acceptable intubation conditions, will be evaluated from four aspects to comprehensively investigate the effectiveness of our interventions.
Single-centre design and lack of blinding among participants and clinicians delivering the intervention.
Introduction
Awake tracheal intubation (ATI) involves placing a tracheal tube in an awake, spontaneously breathing patient, with aim of achieving airway maintenance.1 For elective surgical patients under general anaesthesia induction, intubation after induction is a widely recognised protocol and it is advantageous in some situations. However, endotracheal stimulation may be a second hit on vulnerable patients who are already affected by several anaesthetic drugs. The effects of sedation, hypoxia and changes in intrathoracic pressure can lead to severe haemodynamic instability and hypoventilation at the same time. To this end, ATI with minimal sedation and satisfactory airway regional anaesthesia can reduce the risks of peri-intubation period adverse events.2
Methods of airway regional anaesthesia that facilitate ATI include anaesthesia for the supraglottic and subglottic structures. Translaryngeal injection (TLI) provides anaesthesia to the infraglottic larynx and upper trachea and inhibits cough reflex. Superior laryngeal nerve block (SLNB) can effectively abolish the glottic closure reflex, and blunt the sensation of the structures above the cords. For decades, findings have indicated that a method that consists of SLNB along with TLI can provide satisfactory anaesthesia and intubating conditions, compared with those with topical anaesthesia and TLI.3 4
However, methods of SLNB ranges from various locating techniques to different puncture point, needle orientation and target planes. Among them, ultrasound (US)-guided posterior SLNB is a classical and widely performed method of SLNB, with injection targeted at internal SLN space5 or above thyrohyoid membrane (TH-Mb).6 Inspired by approaches reported by Fowler et al, we present a modified method to achieve bilateral SLNB with a US-guided single injection in the midline. Compared with the method described by Fowler et al, our method not only standardises the exact location of the needle tip via direct visualisation of needle advancement but also provides dynamic monitoring of the spreading of lidocaine. Visualisation of local anaesthetic spread confers advantages such as more accurate estimation of the anaesthesia effect before ATI and avoidance of uncertain attempts. Compared with the traditional US-guided posterior SLNB, our method may be non-inferior for its effectiveness but have better convenience, along with less discomfort and complications, because participants only have to bear one puncture for SLNB and the anterior approach hardly involves nerve and blood vessels.
The objective of this study is to investigate the effectiveness and safety of modified US-guided anterior SLNB for awake videolaryngoscopes-assisted endotracheal intubation in adult elective surgery patients, compared with the traditional US-guided posterior SLNB.
Methods and analysis
Overall study design and timeline
This is a prospective, single-centre, assessor-blinded, parallel-group, randomised, controlled trial designed to evaluate the effectiveness and safety of modified US-guided anterior SLNB to achieve acceptable awake intubation conditions. The protocol is designed and reported according to Standard Protocol Items: Recommendations for Interventional Trials reporting guidelines.7 The study began with participant screening for enrolment in August 2022 with anticipated completion by February 2023.
Consent
Anaesthetist A will perform the introduction to the study, education and consent for all participants. Details in Chinese will be used to introduce the research to participants. Participants can ask questions if anything is confusing. Written informed consent will be obtained after discussion and confirmation that the participants understand the study. The model participant consent form is available as an additional file (). Participants may withdraw from the study at any time.
Participants: inclusion/exclusion criteria
Inclusion criteria
Inclusion criteria are patients scheduled for elective surgeries under general endotracheal anaesthesia in an academic hospital (West China Hospital, Sichuan University), with American Society of anaesthesiologists physical status I–III, aged 18–65 years and agree to sign informed consent.
Exclusion criteria
Patients deemed to have difficult airway (Mallampati grade III–IV, inter incisor distance <3 cm, thyromental distance <6.5 cm or body mass index ≥26 kg/m2); patients with asthma or ischaemic heart disease, patients with SLNB contraindications (cervical movement limitation, cervical mass, bleeding diathesis, allergy to local anaesthetic agents), patients with preoperative hoarseness and sore throat; patients with intellectual impairment or psychiatric conditions and precluding adequate communication; and patients without a plan to extubate immediately after surgery are excluded from the study.
Interventions
Modified US-guided anterior SLNB
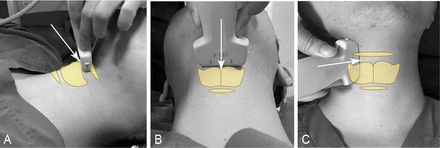
Participants in the modified group will receive the modified US-guided anterior SLNB in a supine position (figure 1A,B). The sniffing position may be required if the exposition of the anterior cervical region is unsatisfied. The probe will be placed in the transverse plane, over the thyroid cartilage (TC). Adjust the midline of the probe until TC is clearly identified in the middle of the screen. Then cephalad move the probe to look for the thyroid incisura notch (the disconnected part of TC) (figure 2A). US-guided out-of-plane injection will be performed in the midline targeting TH-Mb with a 22-gauge 50 mm nerve block needle.8 After confirming the needle tip is in place (thyroid incisura notch anteriorly, TH-Mb posteriorly) and negative aspiration for air or blood, inject 6 mL of 2% lidocaine. The resistance while injecting should be small. Pushing down of TH-Mb and pre-epiglottis by lidocaine provides an early sign of a successful US-guided anterior SLNB (figure 2B).
Probe placement and needle orientation in two methods of ultrasound (US)-guided superior laryngeal nerve block (SLNB). (A) Lateral view of US-guided anterior SLNB. (B) Vertical view of US-guided anterior SLNB. (C) Vertical view of US-guided posterior SLNB.
Transverse sonography of ultrasound (US)-guided anterior superior laryngeal nerve block (SLNB). (A) Transverse scan over the thyroid incisura notch before performing anterior SLNB. (B) After lidocaine injection, the drug pushes the TH-Mb and pre-epiglottis space away, and lidocaine rapidly diffuse to lateral spaces. (C) Left parasagittal scan through the TH-Mb immediately after injection of lidocaine. (D) Right parasagittal scan through the TH-Mb immediately after injection of lidocaine. Arrowheads: TH-Mb, asterisk: air-mucosa interface, white solid arrow: needle orientation, white dotted circled area: lidocaine. Epi, epiglottis; Hy, hyoid bone; PES, pre-epiglottic space; SM, strap muscles; TC, thyroid cartilage; TH-Mb, thyrohyoid membrane.
Traditional US-guided posterior SLNB
Participants in the traditional group will receive the traditional US-guided posterior SLNB in a supine position (figure 1C). The probe will be placed over the submandibular area in a parasagittal orientation. The hyoid bone and TC are identified as hyperechoic structures on sonography. The thyrohyoid muscle and TH-Mb are between these two structures (figure 3A). US-guided out-of-plane injection will be performed with 3 mL of 2% lidocaine anterior to the TH-Mb using a 22-gauge 50 mm nerve block needle.6 The anaesthetist will repeat these interventions on the contralateral side. Pushing down of TH-Mb and pre-epiglottis by lidocaine provides an early sign of a successful US-guided posterior SLNB (figure 3B).
Parasagittal sonography of ultrasound-guided posterior superior laryngeal nerve block (SLNB). (A) Parasagittal sonography before performing posterior SLNB. (B) After injection, lidocaine pushes the TH-Mb and pre-epiglottis space away. Arrowheads: TH-Mb, white solid arrow: needle orientation, white dotted circled area: lidocaine. Hy, hyoid bone; PES, pre-epiglottic space; SLA, superior laryngeal artery; SM, strap muscles; TC, thyroid cartilage; TH-Mb, thyrohyoid membrane.
US-guided TLI
After SLNB, participants will receive a US-guided TLI in a supine position with the neck extended. Place the probe in the transverse plane to obtain a high-bright line echo between the TC and the cricoid cartilage, which is the cricothyroid membrane (C-T Mb) (figure 4). Using out-of-plane visualisation, advance a 22-gauge needle connected to a 5 mL syringe containing 5 mL of 2% lidocaine. Puncture the needle through the C-T Mb. Once air from the tracheal lumen is freely aspirated, place 5 mL of 2% lidocaine. After pulling out the needle, the anaesthetist encourages participants to cough.
Transverse sonography for ultrasound (US)-guided transtracheal block. The sonogram shows the cricothyroid membrane (C-T Mb) and the comet tail artefacts (CTA). The white arrow shows the needle orientation of US-guided translaryngeal block.
Study conductance
Study conductance is displayed in a flow diagram (figure 5). After entering the operation room, the participants are given inhaled oxygen and conventional fluid infusion and monitors will be attached including an ECG, pulse oximeter, non-invasive blood pressure and end-tidal carbon dioxide (EtCO2), to measure mean arterial pressure (MAP), heart rate (HR), and pulse oxygen saturation (SpO2), and EtCO2. Then participants will be induced with 0.03 mg/kg midazolam and 0.1 µg/kg sufentanil. After 3 min, an experienced anaesthetist (anaesthetist A) will perform airway anaesthesia to our participants under aseptic precautions. Participants in the modified group will receive US-guided anterior SLNB. Participants in the traditional group will receive US-guided posterior SLNB. After SLNB, all participants will receive a US-guided TLI. Time taken for airway anaesthesia is defined as the time elapsed from beginning the block procedure (after prepping and draping) until the withdrawal of the needle after TLI. After airway anaesthesia procedures are completed, a piece of clean and dry gauze will be placed to cover needle holes.
Flow diagram of study conductance. SLNB, superior laryngeal nerve block; TLI, translaryngeal injection; US, ultrasound.
Five minutes later, after evaluating the sedation status (Ramsay scale), ATI will be performed by another anaesthesiologist (anaesthetist B) who is skilled in ATI and is blinded to the previous intervention. During the intubation sequence, another independent research assistant (assistant B) will record intubation scores (reported by anaesthetist B), MAP, HR and SpO2. Time taken for tracheal intubation is defined as the time elapsed from insertion of the blade between the teeth to the time the first EtCO2 wave is collected. ‘ATI failed on the first attempt’ is defined as when a patient was unable to cooperate due to grade 4 of reactions or grade 3 of reactions along with closing vocal cords. Patients with unsuccessful ATI received a routine anaesthesia induction and videolaryngoscopes-assisted intubation, and all data were recorded as well as ‘successful’ cases. Recall of intubation and perception of comfort will be evaluated at 30 min following transfer to postanaesthesia care unit (PACU). Severity of postoperative sore throat, hoarseness of voice and injection-site pain will be evaluated at 30 min, 4 hours, 24 hours and 48 hours following transfer to PACU.
Outcome assessments and time points
Primary endpoint measure is the proportion of acceptable intubation condition (AIC). Based on Cormack and Lehane classification during videolaryngoscopy,9 a scoring system presented by Grant and colleagues,10 and 5-point comfort score,4 four variables are reorganised into the intubation condition score (box 1). According to previous guidance,10 when each part was scaled as grade 1 or grade 2, the intubation condition for this participant was defined as acceptable. The proportion of AIC=the number of participants with AIC/number of participants who received ATI in each group.
Intubation condition
Intubation condition grading
A: Cormack and Lehane classification
Grade 1 (the entire glottis is visible)
Grade 2 (posterior commissure of the glottis is visible)
Grade 3 (epiglottis only is visible)
Grade 4 (any portion of the laryngeal structure is invisible)
B: Vocal cord movement
Grade 1 (open)
Grade 2 (moving)
Grade 3 (closing)
Grade 4 (closed)
C: Reaction
Grade 1 (no reaction)
Grade 2 (slight grimacing and/or gagging that do not hinder intubation)
Grade 3 (heavy grimacing and/or gagging that hinder intubation)
Grade 4 (verbal objection)
Grade 5 (defensive movement of head or hands)
D: Unexpected coughing during the process
Grade 1 (none)
Grade 2 (mild)
Grade 3 (moderate)
Grade 4 (severe)
Secondary endpoint measures will include:
The first-attempt intubation success rate=the number of successful first-attempt intubation/ the number of participants in this group.
Haemodynamic changes during ATI: MAP, HR and SpO2 at baseline (T0), immediately before intubation (T1), immediately after intubation (T2) and at 1 min postintubation (T3).
The time taken for airway anaesthesia for each participant will also be recorded using a stopwatch by assistant A. The time taken for awake intubation will be recorded using a stopwatch by assistant B, who is also responsible for the follow-up data collecting.
Recall of intubation will be measured at 30 min following transfer to PACU and graded as (a) no recall, (b) indistinct memories, (c) completely able to recall the whole intubation process.
Participant perception of comfort during intubation will be evaluated at 30 min following transfer to PACU (0–10 scale, with 0 being worst discomfort and 10 being no discomfort).
The perioperative complication rate=the number of participants with perioperative complications in each group/total number of participants in each group. Perioperative complications include severe sore throat and hoarseness (table 1), injection-site pain (0–10 numerical rating scale (NRS), with 0 being no pain and 10 being the worst imaginable pain), airway haemorrhage (defined as blood observed in the endotracheal tube, pharynx, trachea or mouth), SLN damage, local anaesthetic systemic toxicity (LAST), laryngospasm, arytenoid dislocation. Complications will be evaluated at the following time points: 30 min, 4 hours, 24 hours, 48 hours and 72 hours following transfer to PACU.
Grading system for severity of sore throat and hoarseness of voice17
Non-inferiority margin and sample size calculations
Our primary hypothesis is that US-guided anterior SLNB versus US-guided posterior SLNB yield a similar proportion of AICs. Thus, this study will be designed as a non-inferiority trial. To the best of our knowledge, neither the non-inferiority margin for the proportion of AICs nor a placebo-controlled trial is investigated. Since the fixed margin method is not suitable, the non-inferiority margin is comprehensively defined with clinical and statistical significance taken into consideration.
A participant with a comfort score of 0-point (no coughing or gagging in response to intubation) or 1-point (mild coughing and/or gagging that do not hinder intubation) is defined as a participant with AIC.10 This definition is similar to coughing scores described by Zhou et al.11 In their trial, the difference in proportions of participants with 0 or 1 coughing score during bronchoscopy between the US-guided posterior SLNB (2% lidocaine, 3 mL on each side)+cricothyroid membrane puncture (2% lidocaine, 3 mL) versus cricothyroid membrane puncture only (2% lidocaine, 3 mL) is 37.5% (19.4%–53.2%).11 At least preserved fraction of 75% was determined for non-inferiority, based on clinical consideration. Therefore, we assume that a difference in proportion between the modified group and the traditional group less than −4.8% will be considered non-inferiority. Based on a pilot study with 16 participants in each group, the proportion of AICs in the modified group and traditional group was 100% and 93.75%, respectively (unpublished data). The required sample size per group is calculated to be 44, using a one-sided Farrington-Manning test with a margin equal to −4.8%, statistical power of 80% and a one-sided type 1 error rate of 5%. Accounting for at least 10% dropouts, the total sample size is inflated to 100 participants (n=50, per group).
Data collection, management, and analysis
Data collection and management
Data will be collected on paper for each measurement and then electronically recorded by an independent investigator. Once recorded, data will be locked to prevent changes. Missing data because of loss of follow-up will be coded as incomplete. All data collected on paper will be marked with a study identification number to prevent identification of the participant and stored in a locked cabinet. Access to the deidentified data sets will be limited to the study authors.
Data analyses
The proportion of AICs, first attempt intubation success rate, time consumption for intubation and vital signs will be compared for the effectiveness of US-guided anterior SLNB over posterior SLNB. The perioperative complication rate will be compared for the safety of anterior SLNB over posterior SLNB. Time consumption for airway anaesthesia will be compared for convenience of anterior SLNB over posterior SLNB.
For qualitative variables, results will be given in proportion and types, then compared using Pearson’s χ2 test (or Fisher’s exact test). For quantitative variables, results will be reported as mean and SD (or as a median with the 25th and 75th percentiles), then compared using Student’s t-test (or the non-parametric Whitney-Mann U test). No intermediary analysis is foreseen in this study.
The main analysis of differences in the primary outcome measure (the proportion of AICs) will be single-sided using an inferiority test for proportions. The non-inferiority will be declared if the lower limit of the 95% CI of the difference of proportions of AICs (modified group–traditional group) was greater than −4.8%.
Randomisation and blinding
Participants will be randomly allocated into the modified group (modified US-guided anterior SLNB) or the traditional group (traditional US-guided posterior SLNB). Allocation will be performed in a 1:1 ratio using a computerised randomisation table that is generated before participant recruiting by an investigator. An independent assistant, who is not involved in the study, will open a sealed envelope 1 hour before surgery to inform the operator about the block method to be performed. Because of the nature of the trial, the anaesthesiologist performing the block and participant are not blinded to group allocation. However, the outcome assessors are blinded to the intervention.
Monitoring
Adverse events will be collected and recorded only after the participants receive appropriate treatment and intervention until the follow-up study is finished. The Good Clinical Practice Guidelines of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use defined adverse events as any untoward medical occurrence in a study participant that does not necessarily have a causal relationship with the treatment/intervention. Serious adverse events from enrolment until the follow-up study is finished will be reported within 24 hours to the ethics committee and the quality, safety and performance committee.
Prevention and treatment for adverse events
Laryngospasm
Identifying the patients at risk for laryngospasm (upper respiratory tract infection or active asthma) and taking early precautions are the most crucial measures to prevent laryngospasm. Clinical manifestations of laryngospasm include inspiratory stridor; absence of air movement and breath sounds; paradoxical movement of the chest and abdomen; oxyhaemoglobin desaturation, bradycardia and central cyanosis.12 Once laryngospasm is identified, the anaesthetist should identify and remove the offending stimulus, apply a jaw thrust manoeuvre, insert an oral or nasal airway, and give positive pressure ventilation with 100% oxygen. If the obstruction is not relieved, complete laryngospasm should be suspected and the next step should be calling for help and deepening the level of anaesthesia with intravenous or inhalational anaesthetic.
SLN injury
SLN injury may produce vocal cord paralysis in addition to reduced laryngeal sensation, dysphagia and decreased laryngeal cough reflex predisposing to aspiration and impaired vocal quality.13 Actions to reduce the risk of SLN injury include clear identification of superior laryngeal artery, and adjustment of needle access to avoid piercing and damaging the possible area of SLN. Clinical evaluation of SLN injury may include laryngoscopy, strobovideolaryngoscopy and laryngeal electromyography. A wide variety of treatment options have been discussed, ranging from steroids, voice therapy, and a variety of surgical procedures.
Local anaesthetic systemic toxicity
With the absorption of local anaesthetics and their increasing plasma concentration, there is stimulation of the central nervous system followed by depression, tonic-clonic convulsions and respiratory depression. Prevention of LAST in this trial includes clear identification of superior laryngeal artery by using colour Doppler flow imaging, and negative catheter aspiration before injecting lidocaine. Once the LAST is recognised, treatment will be conducted according to the 2020 version of the American Society of Regional Anesthesia and Pain Medicine LAST checklist.14
Ethics and dissemination
The study protocol has been approved by an ethical committee of the West China Hospital (Sichuan University, Chengdu, Sichuan, China), and was registered at the Chinese Clinical Trials Register (www.chictr.org.cn). Important protocol modifications will be communicated to the relevant members of the research team. The eventual trial will be published and subsequently disseminated by West China Hospital. The results of this study will be published in a peer-reviewed journal.
Patient and public involvement
There is no direct patient or public involvement in this study. Patients were not involved in the development of the research question and outcome measures or the design of this randomised controlled trial (RCT).
Discussion
To the best of our knowledge, this is the first study to assess whether US-guided anterior SLNB can be another promising method to facilitate ATI, with non-inferior effectiveness and better safety. We modified the method described by Fowler et al.15 Fowler et al emphasised the feeling of needle entry into the TH-Mb, while we found definite feeling of entry or a loss of resistance only existed in nearly half of the patients. Due to vague needle tip location, there might be much uncertainty involved with the effect of SLNB. We suggested the discrepancy was associated with two reasons: (a) different shapes of needle tips; (b) operatives of SLNB might have various definitions and criteria of feeling the ‘entry’.
Moreover, we found that even if we adjusted the needle tip under TH-Mb, it would still be relatively ‘floating’ away from TH-Mb while injecting lidocaine. Multiple deeper punctures would be required to keep the needle tip beneath TH-Mb, bringing more unnecessary harm.
With the help of ultrasound guidance, we found the feeling of needle entry into the TH-Mb was not a decisive factor of the final effect, which had a stronger correlation with obvious push down of TH-Mb. We chose a target plane that locate just above TH-Mb, which also provided satisfactory effect of SLNB, with a higher level of consistency and quality. In addition, we validated this target plane (above TH-Mb) through left and right parasagittal scan over the TH-Mb immediately after the anterior injection of lidocaine. We found lidocaine spread rapidly to lateral spaces above TH-Mb (figure 1C,D), which indicated that space above TH-Mb was an ideal target plane not only for traditional US-guided posterior SLNB6 but also for our modified US-guided anterior SLNB.
The participants we choose are adult elective surgery patients, and patients with a predicted difficult airway are excluded. The following reasons are considered. (a) The purpose of this trial is to compare the effectiveness of two different airway anaesthesia techniques, rather than the feasibility of ATI in patients with a difficult airway. (b) ATI is not routinely conducted in elective surgery patients, and there is a concern that ATI may bring more unnecessary harm and anxiety. We still consider ATI is the safest way to acquire airway management without worrying about an emergent dilemma between an unexpectedly difficult airway after induction and the absence of spontaneous breathing. Meanwhile, the anxious emotion of participants can be alleviated by proper sedation.16 (c) The modified US-guided anterior SLNB is still a novel method that lacks strong clinical validation. To the full extent of participant protection, we design our inclusion and exclusion criteria to reduce the risk of being caught between a failed ATI and a difficult airway at the same time.
Our study has limitations. One of them is that blinding of participant and intervener is not feasible. To minimise unnecessary harm, this trial is open-labelled with blinded assessors and data analysis. To minimise the bias, we set complete blinding to the anaesthetist who performs ATI. Likewise, evaluation of the primary outcome and most secondary outcomes is completed by a research assistant who is also blinded to the intervention.
If this trial yields clear results, the implications on the clinical practice of ATI could be significant. Performing awake intubation was often complicated and frustrating due to time-consuming procedures and poor patient compliance. With better safety along with non-inferior effectiveness compared with traditional methods, the modified US-guided anterior SLNB will have improvement on both satisfaction of the patient and the anaesthetist while performing ATI. This study may also provide a practical basis for performing ATI with higher quality in difficult airway management.
Supplemental material
Ethics statements
Patient consent for publication
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
YH and QH are joint first authors.
YH and QH contributed equally.
Contributors Study design/planning: QH, YH, GC. Pilot study implementation: YH, TZ. Data analysis: QH, TZ. Writing paper: YH, QH. Revising paper: all authors.
Funding This work was supported by the National Key R&D Programme of China [grant number 2018YFC2001800] to TZ; and CAMS Innovation Fund for Medical Sciences [grant number 2019-I2M-5-011] to TZ.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.