Article Text
Abstract
Objectives In the care of coronary artery disease (CAD), evidence questions the adequate application of guidelines for cardiovascular procedures, particularly coronary angiographies (CA) and myocardial revascularisation. This review aims to examine how care providers’ guideline adherence for CA and myocardial revascularisation in the care of chronic CAD was assessed in the literature.
Design Scoping review.
Data sources PubMed and EMBASE were searched through in June 2021 (rerun in September 2022).
Eligibility criteria We included studies assessing care providers’ adherence to evidence-based guidelines for CA or myocardial revascularisation in the care of chronic CAD. Studies had to list the evaluation of guideline adherence as study objective, describe the evaluation methods used and report the underlying guidelines and recommendations.
Data extraction and synthesis Two independent reviewers used standardised forms to extract study characteristics, methodological aspects such as data sources and variables, definitions of guideline adherence and quantification methods and the extent of guideline adherence. To elucidate the measurement of guideline adherence, the main steps were described.
Results Twelve studies (311 869 participants) were included, which evaluated guideline adherence by (1) defining guideline adherence, (2) specifying the study population, (3) assigning (classes of) recommendations and (4) quantifying adherence. Thereby, primarily secondary data were used. Studies differed in their definitions of guideline adherence, where six studies each considered only recommendation class I/grade A/strong recommendations as adherent or additionally recommendation classes IIa/IIb. Furthermore, some of the studies reported a priori definitions and allocation rules for the assignment of recommendation classes. Guideline adherence results ranged from 10% for percutaneous coronary intervention with prior heart team discussion to 98% for coronary artery bypass grafting.
Conclusion Due to remarkable inconsistencies in the assessment, a cautious interpretation of the guideline adherence results is required. Future efforts should endeavour to establish a consistent understanding of the concept of guideline adherence.
- coronary heart disease
- coronary intervention
- quality in health care
- cardiology
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
A robust methodology including a systematic literature search and data extraction conducted in duplicate.
This review synthesises the methods used to assess guideline adherence by summarising the four main steps of guideline adherence measurement.
Due to the absence of a validated instrument and focusing on examining the methods used to assess guideline adherence, no quality assessment of the methods used to measure guideline adherence could be conducted within this scoping review.
Introduction
Coronary artery disease (CAD) is one of the most important widespread diseases,1 and still the major cause of mortality at the global level.2 With a lifetime prevalence of 8%1 and a proportion of 16% of global deaths,2 CAD is associated with a significant economic burden for healthcare systems all around the world.3
In order to improve the quality of CAD care, which is highly complex and varied in nature, many national and international scientific societies have developed evidence-based clinical practice guidelines.1 4 5 By systematically providing the best evidence available, these guidelines aim to support health professionals in clinical decision-making and promote high-quality care.4 6 Furthermore, due to concerns surrounding excessive utilisation of tests and procedures, appropriate use criteria (AUCs) have been developed in an effort to improve appropriate resource utilisation by providing a consensus judgement on the utility of a test or procedure in specific clinical scenarios. However, AUCs are derivations from the guidelines, and the guidelines remain the primary source of guidance for clinicians.7
Although there are established strategies for disseminating and implementing evidence-based guidelines in clinical practice,8 there is still some question as to whether guidelines for cardiovascular procedures, in particular, those for coronary angiography (CA) and myocardial revascularisation (eg, percutaneous coronary intervention (PCI)), are being applied adequately.9 10
There has been growing interest recently in evaluating the uptake among healthcare providers of clinical practice guidelines for patient treatment in chronic CAD care, that is, the adherence of healthcare providers to clinical guideline recommendations.11–14 Since evidence on guideline adherence in clinical practice contributes to quantifying the quality of care15 and may be used to stimulate activities that promote a more guideline-adherent use of cardiovascular procedures,14 it is important to ensure that the concept of guideline adherence is measured accurately and consistently. To the best of our knowledge, there is no available evidence on the accuracy and comparability of the methods used to assess guideline adherence for invasive procedures in the field of chronic CAD care. The aim of this scoping review is thus (1) to examine the methods and results of studies that assessed guideline adherence for invasive diagnostic and therapeutic procedures in patients with chronic CAD and (2) to compile the general steps used to assess guideline adherence.
Methods
We performed a scoping review of methods used to assess guideline adherence for invasive diagnostic and therapeutic procedures in chronic CAD. The review was reported according to guidance in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews Statement.16 The review was not registered, and no protocol was published. The study selection process was conducted in duplicate (HK and YS). In case of disagreement, a third reviewer (DM) was consulted. Two reviewers (HK and YS) performed subsequent data extraction using standardised extraction forms.
Literature search
We conducted the search in the bibliographic databases PubMed and EMBASE (via Elsevier) using the search strategies presented in online supplemental file 1. Following removal of duplicates, studies were selected by examining the eligibility criteria stated below. The titles and abstracts were screened, and potentially relevant studies were subjected to a full-text review. In addition to this, cross-references and similar articles from the included articles were checked for inclusion. The search was conducted in June 2021 (and repeated in September 2022).
Supplemental material
Eligibility criteria
We selected studies that assessed guideline adherence among healthcare providers for invasive diagnostic or therapeutic procedures in the field of CAD care: CA, PCI and coronary artery bypass grafting (CABG). Guideline adherence was defined as practitioners’ decisions following clinical practice guidelines.14 Thus, in this review, results presented as ‘adherent care’, ‘compliant care’,14 ‘care in agreement with the guidelines’ and ‘appropriate care’ were included and summarised under the term ‘adherent care’. In order to be considered, the studies had to be published in German or English, list the evaluation of guideline adherence as one of the respective study’s objectives, and include a description of the evaluation methods used. In addition to this, the studies had to include patients with chronic CAD and report the corresponding results on guideline adherence. Furthermore, the studies had to list the specific guidelines and recommendations used as a basis for their assessment of adherence. Since evidence-based guidelines are the primary source of guidance for physicians,7 the search only included studies that addressed adherence to this type of guidance.
Publications that focused on other decision aids, such as AUCs or performance measures, were excluded because these are derivatives from clinical practice guidelines.7 Unlike evidence-based guidelines, performance measures aim to operationalise guideline recommendations, whereas AUCs only supplement guideline recommendations using specific clinical scenarios.7 In addition to this, literature reviews and study protocols were excluded.
Extraction and synthesis of data
Data on the main characteristics of the studies and their results were extracted (for consistency, the results of all the studies are presented in terms of adherence rather than non-adherence). In order to describe the methods used to assess guideline adherence in the field of chronic CAD care, we extracted information relating to the methodological aspects assumed to affect the assessment of guideline adherence,17 that is, data source and collection, data variables, the study’s definition of guideline adherence and the quantification method used. In addition to this, information regarding the underlying guideline recommendations and the target procedure/population was also extracted. Based on these factors, we summarised the main steps used to assess guideline adherence. Since most of data extracted were qualitative in nature, a narrative synthesis was conducted.18
Results
Literature search
The search yielded 1384 publications. Following the removal of 252 duplicates, a total of 1132 titles and abstracts were screened and 79 potentially relevant studies were subsequently subjected to a full-text review. Based on the eligibility criteria, 67 of these studies were excluded. As the screening of cross-references and similar articles did not identify any additional relevant publications, 12 studies were ultimately included in this review (see flowchart in figure 1 and online supplemental file 2 for details of the excluded studies).
Supplemental material
Flowchart for the literature search.
Study characteristics
Three of the 12 studies included in the review assessed guideline adherence for the invasive diagnostic CA,19–21 while nine did so for therapeutic revascularisation by means of a PCI/percutaneous transluminal coronary angioplasty (PTCA) and/or CABG.22–30 With one exception, all the studies were either based on a retrospective cross-sectional design (n=7)21 22 25–27 29 30 or a prospective cohort design (n=4).19 20 24 28 The studies evaluated both primary and specialised care (eg, catheterisation laboratory) over study periods ranging from 5 months19 to 5 years27 from 199122 23 to 2020.20 The study populations varied with regards to care setting, disease state, prior treatment and patient demographics. An overview of the study characteristics is provided in online supplemental file 3.
Supplemental material
Assessment of guideline adherence
Methods and results
The majority of the studies (n=11) evaluated adherence to the guidelines published by the American College of Cardiology (ACC)/the American Heart Association (AHA) and the European Society of Cardiology. Specifically, the studies assessed adherence to recommendations on the performance of a revascularisation in general,23 30 a CABG,22 24 29 a PCI/PTCA,22 24 25 27 an ad hoc PCI,25 26 a PCI with prior heart team discussion26 28 and a CA.19–21
Most of the studies were based on secondary data from registries,28–30 patient records21–26 or administrative data.22 23 27 However, two studies were based on primary data obtained from prospective records of consecutive patients (eg, severity of stenosis, symptoms, procedures).19 20 Eleven of the studies used clinical data variables, including information regarding the extent of CAD, the patients’ symptoms, the diagnostic test results, the clinical history, risk factors and treatments provided.19–26 28–30 In one study, specific procedure codes and diagnoses within the utilised claims data were resorted.27
The studies’ definitions of guideline adherence were based on recommendation classes/grades (used in USA, German and European guidelines) or levels of recommendation strengths (used in British guidelines). Recommendation classes/grades or levels of strengths indicate an estimate of the size of treatment effect that takes into account risks and benefits and evidence of and/or agreement on the effectiveness of a procedure.31 32 In particular, the USA and European guidelines are based on three classes of recommendation: (1) class I=procedure is recommended, (2) class II=conflicting evidence/agreement; procedure is reasonable/should be considered (IIa) or may be reasonable/considered (IIb) or (3) class III=procedure is not recommended.33 34 Similarly, the German guidelines categorise recommendations using three grades: (1) grade A=procedure shall (not) be performed, (2) grade B=procedure should (not) be performed or (3) grade 0=procedure could be performed.35 In British guidelines, strong recommendations are applied where there is clear evidence of a benefit (ie, ‘offer’), while a less certain recommendation indicates that the evidence of a benefit is less certain (ie, ‘consider’).36
All the studies determined guideline adherence on an individual basis for each patient and summed it up across the study population. Adherence was quantified using a nominal measure, either binary (adherent/non-adherent treatment),19 20 23–28 30 multicategorically (useful/justified, uncertain and not useful/not indicated procedures)21 or a combination of the two.22 29
The extent of guideline adherence depended on the procedure in question, and ranged from: 67% to 91% for PCI/PTCA,22 24 25 27 17% to 20% for ad hoc PCI,25 26 10% to 19% for PCI with prior heart team discussion,26 28 49% to 98% for CABG,22 24 29 40% to 94% for revascularisation in general23 30 and 52% to 79% for CA.19–21 An overview of the methods used to assess guideline adherence is presented in table 1 (for detailed information, see online supplemental file 4).
Supplemental material
Methods
Main steps used to assess guideline adherence
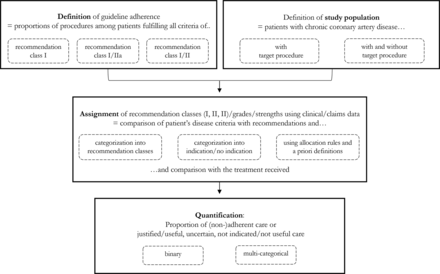
Four steps for assessing guideline adherence were identified, the first two of which could be undertaken simultaneously (see figure 2).
Main steps used to assess guideline adherence.
Definition of guideline adherence
In all of the studies, guideline adherence was defined as the proportion of procedures among patients who fulfilled all the criteria for a specific recommendation (class). The recommendations used in the studies varied. Several of the studies limited their definitions of adherent care to procedures corresponding to recommendation class I/grade A/strong recommendations (ie, ‘is recommended’),20 23 26–28 30 while others additionally considered recommendation class IIa (ie, ‘is probably recommended’),21 24 25 or even recommendation class IIb (ie, ‘might be considered’)19 22 29 to be adherent.
If the criteria for a specific recommendation (class) were not fulfilled, some of the studies additionally defined guideline-adherent care as ‘doing nothing’.20 23 27 30 Non-adherent care reflected both procedures offered to patients without a corresponding recommendation and cases where no procedure was performed despite revascularisation or diagnostic CA being recommended.
Definition of study population
While eight of the studies only considered patients who received a specific target procedure,19 21 22 24–26 28 29 four included patients regardless of what treatment they had received in order to examine guideline adherence for revascularisation or diagnostic CA.20 23 27 30
Assignment of recommendations and recommendation classes/grades/strengths
Using clinical data collected from different sources (see table 1), for each patient, it was checked (1) which class of recommendation or (2) whether the specific recommendation (class) under evaluation matched the patients’ disease criteria (eg, symptoms, severity of disease). Six of the studies categorised patients into recommendation classes I, II (a,b) and III.19 21–23 25 29 The remaining studies focused on specific recommendations or recommendation classes (eg, recommendation class I30) and merely categorised patients into two groups: ‘procedure indicated’ or ‘procedure not indicated’.20 24–28 30 Whether or not the care in question was guideline-adherent was ultimately determined by comparing the results of the assignment with the treatment received. For example, a PCI for a patient with a recommendation class I for PCI was considered adherent.
Overall, there were differences in terms of how the studies dealt with ambiguous assignments and cases of insufficient information for an explicit assignment of recommendation classes. Only one study reported a prespecified allocation rule for cases of an ambiguous assignment (ie, where a patient was assigned to more than one recommendation class).27 In cases where guideline criteria had not been explicitly defined, four studies used a priori definitions of these criteria for an explicit assignment (eg, evidence of ischaemia, morbidity risk).22 23 29 30
Quantification of guideline adherence
Estimating the proportions of patients with adherent or non-adherent care, nine of the studies used a binary approach.19 20 23–28 30
Three of the studies quantified the results according to the considered guidelines using a multicategorical approach, reporting the proportions of procedures within each recommendation class that were defined as justified/useful (class I), uncertain (class II) and not indicated/not useful (class III).21 22 29 Of these three studies, one adapted this rating to its own definition by quantifying adherent (class I and IIa), uncertain (class IIb) and non-adherent (class III) procedures.21 The other two studies used an additional binary categorisation into adherent and non-adherent care by accordingly assigning the cases that had initially been classified as uncertain.22 29
Discussion
To the best of our knowledge, this is the first scoping review to summarise the methods used to assess guideline adherence in studies that evaluate invasive diagnostic and therapeutic procedures in patients with chronic CAD. Based on 12 studies investigating physicians’ adherence to European, USA, German and British guidelines, we examined methods and results and identified the main steps used to assess guideline adherence. The studies included in the review used similar approaches to evaluate guideline adherence, that is, (1) defining guideline adherence, (2) specifying the study population, (3) assigning recommendations or recommendation classes/grades/strengths and (4) quantifying guideline adherence. However, differences were identified with regards to data sources and collection, the definition of guideline adherence, the assignment of recommendation classes/grades/strengths and the results on guideline adherence.
Data sources and collection
Although two of the studies prospectively collected primary data,19 20 most used secondary data that had been collected retrospectively.21–30 Even though secondary data often represent a more easily accessible and affordable data source, they are usually not collected for the purpose of assessing guideline adherence. As a result, the database may be non-specific (ie, information is available on a more aggregate level without providing clinical details) or incomplete (ie, required information is missing entirely).37 This limits the informative value of the database, particularly given the complexity of treatment decisions.
Furthermore, the accuracy of information obtained from patient records, registries and claims data is highly dependent on the standard and quality of the documentation of the care providers.15 38 In particular, the interpretation and documentation of patients’ test results (eg, extent/significance of coronary stenoses) and symptoms (eg, type of chest pain), which are key criteria for the assignment of recommendation classes, vary widely.19 20 24 25 29 39 Moreover, secondary data often fail to provide information on contraindications or patient preferences that could justify deviations from the guidelines.22–24 The appropriateness of claims data for assessing guideline adherence might additionally be affected by factors such as the complexity of coding or economic incentives (eg, coding higher disease severity in order to generate higher payments).40
Overall, these issues might have led to misclassification or exclusion of patients and procedures,15 22 23 26 29 30 and, thus, contributed to a potential overestimation or underestimation of guideline adherence.22 23
A prospective collection of primary data alone or in combination with secondary sources (as reported in two studies19 20) may represent the first step towards obtaining a more reliable database. In addition to this, a priori definitions of all variables in order to ensure objective data collection, measures for ensuring data completeness and methods for handling missing data are requirements for an explicit assignment.
Definition of guideline adherence
Half of the studies only considered recommendation class I/grade A/strong recommendations to be adherent,20 23 26–28 30 while the others also included recommendation classes IIa and IIb. This difference has a significant impact on the overall results regarding guideline adherence and its interpretation and comparability. For example, excluding recommendation class II would decrease guideline adherence by 11%–12% in two of the studies, which assessed CABG,22 29 and by 58% in one study that assessed PCI.22 The recommendation classes I/strong recommendations20 22 23 26–28 30 and IIa21 24 25 are based on high-level evidence, which is associated with a strong or intermediate positive benefit–risk estimate.7 In contrast, recommendation class IIb as a guideline-adherent scenario19 22 29 is only associated with a marginal benefit–risk ratio or uncertain outcomes.7 As such, an assessment of the impact of addressing different classes of recommendation on guideline adherence (eg, by means of sensitivity analyses) would be appropriate.
Assignment of recommendation classes/grades/strengths
The differences found in the assignment of recommendation classes/grades/strengths relate to the use of a priori definitions of guideline criteria and allocation rules (explicitly assigning each patient to one recommendation (class)). Five of the studies only used these in case of difficulties in the interpretation of guideline criteria or an ambiguous assignment.22 23 27 29 30 A priori definitions and allocation rules ensure a more objective and explicit assignment of recommendation classes/grades/strengths. However, different interpretations of assignment criteria and allocation rules in clinical practice and research are likely to affect the measurement of guideline adherence. A consistent understanding of the guideline criteria for clinical implementation and research could be achieved by further establishing the clinical standard criteria developed by the ACC/AHA. The application of these criteria would aim to harmonise cardiovascular terminology, thus enabling improved clinical communication and facilitating research.41
Results on guideline adherence
The study results differ in the extent of guideline adherence, particularly between studies that did not examine the same treatment decisions. The lowest extent of adherence was observed for a PCI with prior heart team discussion (10%)26 28 and an ad hoc PCI (17%),25 while the highest extent of adherence was observed for CABG (98%).29 Since a high level of evidence has a positive impact on the implementation of guidelines in clinical practice,8 22 this variation might be explained by the low level of evidence for the recommendations for PCI with prior heart team discussion and ad hoc PCI (ie, consensus of experts or small/retrospective studies and registries).33 42 43 The providers’ explanations and the patients’ perceptions regarding the benefits and risks of the procedures in question may also contribute to this variation.44 Patients may frequently request a PCI due to the invasiveness of CABG and the higher value assigned to the short-term benefit of PCI when compared with the long-term advantages of CABG.44 This might lead to a lower adherence for (ad hoc) PCI.
Those studies that examined the same treatment decision showed less variation than those that evaluated different treatment decisions. The extent of adherence varied least for an ad hoc PCI (between 17% and 20%)25 26 and most for revascularisation in general (between 40% and 94%).22 24 29 In these studies, the observed variation may be the result of methodological differences (eg, different data sources or different definitions of guideline adherence).
Guideline adherence may also differ in the time of development and the temporal consistency of guideline recommendations. For example, the lowest extent of guideline adherence was observed for recommendations developed in 201024 45 (ie, heart team discussions before PCI and revascularisation decisions based on the Syntax Score24 26 28) and for recommendations that changed significantly over time46 (ad hoc PCI26). This might indicate difficulties in the implementation of the evolving and more complex recommendations over time.8 However, the heterogeneity of the included studies did not allow an analysis of a temporal trend.
Furthermore, the variation of results may be influenced by external factors.8 For example, initiatives to improve the quality and cost-effectiveness of care using decision aids (eg, AUCs and performance measures) and financial incentives to encourage compliance with guidelines (eg, pay-for-performance models) are well established in the USA7 47 and may have improved awareness of clinical guidelines among providers.48
In addition, guideline adherence results vary in terms of the interpretation of non-adherence. Because in most of the studies only the proportion of patients receiving a procedure without a corresponding indication was reported, the derived non-adherence could be primarily interpreted as potential overuse. However, both overuse and underuse of medical procedures reduce quality of care.49 Therefore, to assess the proportion of patients not receiving a procedure with an indication (as reported in two studies23 30) would also be informative for developing targeted interventions to promote high quality care.
Some efforts will be needed in order to advance research on guideline adherence and improve the credibility of the results. First, prospective databases that comply with guideline criteria should be developed for an objective collection of relevant clinical data. Second, the establishment and use of consistent definitions for guideline criteria (eg, the clinical standard criteria published by the ACC/AHA) should be promoted in care and research. Third, in order to facilitate an adequate interpretation of results, we highly recommend the development of reporting standards for studies that evaluate guideline adherence.
Limitations
This review should be interpreted in the context of the following limitations. First, the literature search was performed in two databases and was limited to studies available in German or English, so other studies relevant to the review may have been overlooked. However, this may only have a minor impact on the results of this review, as the screening of the reference lists of the studies included in the search did not yield additional methods.
Second, due to the absence of a validated instrument, it was not possible to conduct a quality assessment of the methods used to measure guideline adherence. However, since the primary objective of this review was to examine the methods used to assess guideline adherence, this might likely not affect the results of this review.
Third, most of the included studies were retrospective in design and used secondary data, so the credibility of the guideline adherence results is limited. However, we extensively discussed these methodological aspects among others to enable readers to adequately interpret results on guideline adherence.
Conclusion
We observed inconsistencies in the assessment that limit the credibility and comparability of the guideline adherence results. For researchers, the four assessment steps identified in the review may serve as orientation for ensuring consistency. However, the data collection, the definitions, the assignments of recommendations and the methods of quantification require further standardisation. Since evidence on guideline adherence may be used to set up tailored interventions in clinical practice patterns in efforts to improve care, the available evidence regarding guideline adherence should be interpreted with caution. As such, future efforts should endeavour to establish a consistent understanding of the concept of guideline adherence.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors HK, YS and DM were involved in the conception and design of this review. The selection of articles was carried out by HK and YS, consulting DM as third reviewer in case of disagreement. The data extraction and analysis were conducted and guided by HK and YS. All the authors contributed to the data interpretation. HK and YS wrote the final manuscript. BW, DM and SS critically revised the final manuscript. All the authors read and approved the final manuscript. YS is responsible for the overall content as guarantor.
Funding This work is financed by the Innovation Committee at the Federal Joint Committee (Gemeinsamer Bundesausschus (GBA) [grant number 01VSF17011]; Erfassung undOptimierung der Leitlinienadhärenz im Indikationsstellungsprozess zur Herzkatheteruntersuchung bei stabiler Koronarer Herzerkrankung (ENLIGHT-KHK) [to YS, BW, and DM]).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.