Article Text
Abstract
Objectives To evaluate through a systematic review the effectiveness of electronic methods in monitoring adherence to regular inhaled corticosteroids (ICS) alone or in combination with long-acting β2-agonists (LABAs) and their effect on clinical outcomes.
Design A narrative systematic review.
Data sources MEDLINE, EMBASE, Cochrane Database of Systematic Reviews and Web of Science were searched through up to 10 July 2022.
Eligibility criteria We included peer-reviewed studies of qualitative and quantitative outcomes that compared the effect of electronic methods to routine non-electronic monitoring intervention or placebo among children and adults with asthma on medication adherence rates to regular ICS alone or in combination with LABA, asthma control and asthma exacerbations.
Data extraction and synthesis Data extraction was performed according to a predetermined sheet specific to the review objectives. The risk of bias was assessed using the Cochrane Risk of Bias Tool for randomised controlled trials and the Risk of Bias in Systematic Reviews tool for systematic reviews. Meta-analysis was not possible based on the findings of the scoping search; however, a narrative review was performed to allow for the grouping of results based on asthma inhaler adherence rates, asthma control and exacerbations.
Results Six articles comprising 98 studies published from 1998 to 2022 in the USA, Canada and the UK were included. Compared with the control, electronic monitoring devices (EMDs) showed a 23% adherence improvement, mean difference (MD) of 23%, 95% CI 10.84 to 34.16, p=0.0002. Asthmatic children were 1.5 times more likely to be adherent using EMDs compared with non-EMD users (RR=1.5, 95% CI 1.19 to 1.9) (p<0.001). Mobile devices and text message reminders (MHealth) showed a 12% adherence improvement (MD 12%, 95% CI 6.22 to 18.03) (p<0.0001), alongside a small to medium improvement in asthma control (standardised mean difference (SMD) 0.31, 95% CI 0.17 to 0.44), small improvement in asthma-related quality of life (SMD 0.26) (p=0.007) and variable risk reduction in asthma exacerbations for digital health (risk ratio 0.53, 95% CI 0.32 to 0.91) (p=0.02) compared with EMDs, which showed insignificant differences (risk ratio 0.89, 95% CI 0.45 to 1.75) (p=0.72). Technologies combined yielded variable adherence effects, with an SMD for eHealth of 0.41, 95% CI 0.02 to 0.79, and MD for digital health was 14.66% higher than the control, 95% CI 7.74 to 21.57. Heterogeneity between studies was significant (eHealth I2=98%, digital I2=94%).
Conclusion Electronic methods improved adherence to inhaled medications in asthma. EMDs appear to be the most effective technology, followed by mHealth. The adherence improvement was associated with a small clinical improvement. There was inconsistent overlapping of terminology describing electronic methods that require standardisation. Data on the cost-effectiveness of electronic devices and their utilisation in severe asthma are lacking and require further research.
PROSPERO registration number CRD42022303069.
- Asthma
- Telemedicine
- Information technology
Data availability statement
No data are available.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines for systematic reviews.
Benefited from the multidisciplinary expertise of a lead in severe asthma service, pulmonologists and clinical pharmacists, evaluating and comparing the studies.
Used Cohen’s d to compare different effect estimates of multiple studies that used various adherence assessment tools in monitoring adherence as an outcome since standardised mean difference alone tends to overestimate the effect size, especially with small sample size studies.
Not a meta-analysis.
Only two of the five identified systematic reviews were registered on PROSPERO, highlighting a need to avoid duplicating work through protocol registration.
Background
Asthma is a common chronic disease characterised by chronic airway inflammation with a history of respiratory symptoms that vary over time. It is prevalent, affecting up to 18% of the population globally.1
Patient adherence to treatment is defined as using therapy as agreed with the healthcare professionals (HCP).2 Uncontrolled asthma has significantly increased healthcare utilisation and costs.3 The estimated unused medicines’ cost in the National Health Service in the UK is around £100 million annually.4 It has been estimated that 30%–50% of children and adults with asthma fail to use medications as directed.5 6 Poor adherence to asthma medications can lead to asthma exacerbations, worse health outcomes, hospitalisations, higher mortality and increased healthcare utilisation. Non-adherence to regular inhaled corticosteroids (ICS) alone or in combination with a long-acting β2-agonist (LABA) contributes to 34% of asthma deaths in the United Kingdom.7 Treatment adherence can be monitored subjectively using validated questionnaires, or objectively by using different methods, including drug dose counting, prescription possession ratios and measuring drug levels in the blood or urine.8
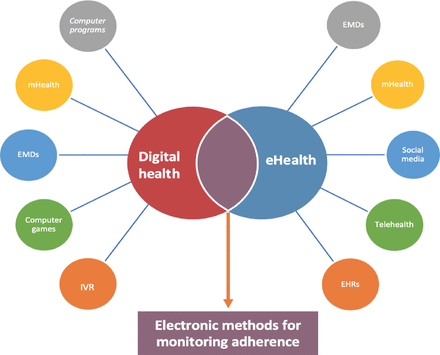
Electronic methods offer a potential solution to improving adherence to asthma medication. The WHO’s definition of ‘eHealth’ is the use of health information and communication technologies (ICT) that include treatment, research, education of HCP, public health monitoring and a variety of technological interventions. The umbrella of eHealth includes Telehealth (telephonic or electronic technology for long-distance healthcare monitoring) or electronic monitoring devices (EMDs) (eg, a propeller that includes a sensor and mobile app), mHealth (clinical intervention by mobile devices and text message reminders) and social media (incorporating an interactive web-based platform).9 Digital health is a new term that includes electronic interventions for health and innovative forms of ICT to address health needs. Digital health contributes to monitoring adherence that is highly customisable low cost and easily accessible. The terms eHealth and digital health are often used interchangeably. However, their intended meaning may vary. eHealth refers to the provision of high-quality care for an increasing number of people and doing so cost-effectively and efficiently. Digital health indicates the use of electronic tools to address health needs and is considered the umbrella label for a wide range of technological interventions that could meet the healthcare challenges of the present consumer-driven to include digital consumers.10 11
Electronic methods can improve adherence to asthma medications, which may not necessarily translate to improved clinical outcomes.12 Electronic methods of monitoring patients with asthma have increased rapidly in the last decade, particularly during the COVID-19 pandemic. However, their effectiveness and utility in asthma remain uncertain. Electronic methods may reveal different outcomes such as improved adherence and asthma control or poor adherence and poor control in which case adherence improvement will be required. However, in cases of persistent poor asthma control despite good adherence, treatment step-up, including initiation of biologic treatment in severe asthma will be required to improve asthma outcomes and control.13
In this systematic review, published peer-reviewed studies were examined to provide the best current evidence on the use of electronic methods compared with standard therapy (without electronic technology). Since the optimal method for monitoring adherence to regular ICS alone or in combination with an LABA remains unclear, this study aimed to evaluate the effectiveness of electronic methods in monitoring and enhancing adherence to regular ICS alone or in combination with an LABA and any consequent effect on asthma clinical outcomes.
Objectives
To conduct a systematic review to identify and evaluate the current published peer-reviewed studies on various electronic methods used to monitor adherence to regular ICS alone or in combination with LABA in adults and children with asthma.
To assess the effectiveness of various electronic methods in monitoring the adherence to regular ICS alone or in combination with LABA versus conventional care or placebo by comparing the mean difference of medication adherence rates.
To compare the various electronic methods to monitor the adherence to regular ICS alone or in combination with LABA with changes in adherence rates and associated asthma-related clinical outcomes, such as asthma control, asthma exacerbations, emergency visits or oral corticosteroid use.
To provide an evidence-based recommendation for the optimal electronic method/s for monitoring adherence to regular ICS alone or in combination with LABA by comparing the performance of published electronic methods to conventional care or placebo.
To identify and report on current gaps in the literature on the use of these technologies and recommend future research requirement.
Methods
Design
A narrative systematic review.
Setting
There were no boundaries by type of setting.
Study eligibility criteria
Study design
A narrative systematic review including papers with either or both qualitative and quantitative outcomes.
Inclusion criteria
All eligible published peer-reviewed studies not in exclusion criteria were included with no restrictions on the study design, or language to minimise bias while collating and synthesising evidence from all the relevant literature.
Exclusion criteria
Abstract-only articles, articles not reporting research design or methodologies and descriptive/editorials/ opinion articles. Multiple reports of the same study included in the systematic reviews were excluded before the data collection process.
Participants
The study included children and adults (age range of 2–98 years) with a confirmed diagnosis of asthma of any type or grade as defined by the Global Initiative for Asthma guidelines who are prescribed regular ICS alone or in combination with a LABA.
Interventions
Interventions of interest included electronic methods with/without an audio-visual reminder function, online apps, short message service reminder functions or data recording or any additional electronic intervention, which allows HCP to provide adherence feedback. Studies using electronic methods to measure adherence for non-electronic adherence interventions were not considered.
Comparators
For patients prescribed regular ICS alone or in combination with an LABA, reports involving their routine non-electronic monitoring intervention or placebo groups without monitoring adherence were used as comparators.
Primary outcomes
The primary outcomes of interest were the effect of electronic methods on medication adherence rates to regular ICS alone or in combination with an LABA, asthma control (measured using clinically validated questionnaires, eg, asthma control test (ACT) or asthma control questionnaire (ACQ)) and the number of asthma exacerbations as defined by hospital admissions or treatment with oral corticosteroids.
Secondary outcomes
The secondary outcomes involved exploring the effect of electronic methods on the forced expiratory volume (FEV1), peak expiratory flow rate, fraction-exhaled nitric oxide (FeNO), days of missed school or work, cost of interventions, patient satisfaction and adverse events/side effects.
Study appraisal and synthesis methods
This systematic review was completed according to a predetermined protocol with prespecified eligibility criteria to identify information relevant to the research question and associated study objectives. The study protocol was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-P statement and registered in the International Prospective Register of Systematic Reviews (PROSPERO) database. The present systematic review is reported using the PRISMA Checklist (online supplemental appendix 1).
Supplemental material
Databases
The databases included were MEDLINE (OVID interface, 1948 onwards), EMBASE (OVID interface, 1980 onwards), Cochrane Database of Systematic Reviews and Web of Science. The decision to use these sources was agreed by a group of asthma experts and a professional librarian at the University of Birmingham (UK) to ensure comprehensive outputs. To maximise the search results, all published studies were searched without time or language limitations, and output reference lists were inspected for additional relevant studies. Authors’ personal files were also examined to collect all relevant studies. Rayyan software14 was used to screen the titles and abstracts of identified studies based on the eligibility criteria. Studies were grouped according to their outcome in a tabulated form to allow for semiqualitative comparisons. All results were reported in the context of overall study quality.
Search strategy
A three-step comprehensive search strategy was conducted to identify peer-reviewed studies comparing the effectiveness of electronic methods compared with conventional care or placebo in monitoring the adherence to regular ICS alone or in combination with a LABA. Initially, MA suggested predefined search terms and combinations with database-specific standard vocabulary based on the indexing methodology used by each specific database (online supplemental appendix 2). A systematic and comprehensive literature search was then conducted using MEDLINE, EMBASE, Cochrane Database of Systematic Reviews and Web of Science combining three concepts: asthma, adherence and electronic. A second step involved consulting a group of asthma experts and professional librarians at the University of Birmingham (UK) to further develop the search strategy. The resultant strategy was used to conduct the systematic review: an update was conducted before data synthesis in July 2022 to ensure that the maximum number of relevant outputs were retrieved.
Study records
Data management
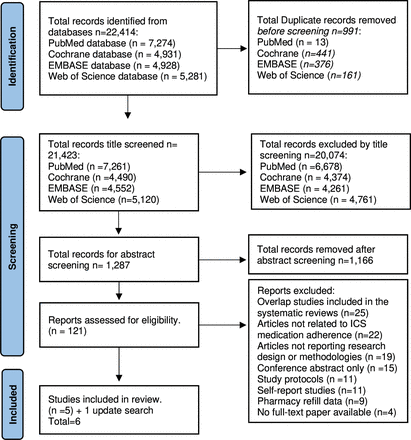
Searches were downloaded and duplicates were removed using Zotero V.5.0 software. Two researchers (MA and AM) independently screened titles and abstracts and assessed studies for inclusion against eligibility criteria. Potentially eligible studies were ordered as a full text and reviewed independently by the primary researcher (MA). Disagreements were referred to a third researcher (JFM). The numbers of studies included and excluded at all stages are shown in figure 1.
Study identification and selection process. The flow of information through the different stages of the systematic review and meta-analysis according to PRISMA guidelines. ICS, inhaled corticosteroids; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Selection process
Data collection process
Data extraction was conducted by the primary researcher (MA) and checked and agreed on by two researchers (AM and JFM). Data extraction was performed according to a predetermined data extraction sheet specific to the review objectives (online supplemental appendix 3). The predetermined data extraction table was reviewed and agreed on by two researchers before use. For consistency and clarity, differences were resolved at a consensus meeting of all authors.
Data items
Extracted data included the study description, search strategy, intervention, comparator, outcome measures, risk of bias, study findings and any additional information (online supplemental appendix 4). One researcher completed data extraction (MA) and a second researcher cross checked the results (AM). Discrepancies were cross checked by a third researcher (JFM) to reach a consensus agreement.
Data synthesis
Meta-analysis was not possible based on the findings of the scoping search; however, a narrative systematic review was performed to allow for grouping of results based on asthma inhaler adherence rates, asthma control and exacerbations.
Standardised mean difference
The standardised mean difference (SMD, Cohen’s d) was used to provide an estimate of effect of pooled data from multiple studies using different tools to measure outcomes of interest. SMD tends to overestimate the effect size, especially when the sample size is small (<20). SMD values of 0.2, 0.5 and 0.8 represented small, medium and large effects, respectively. If two normally distributed populations were equal in size and variability, then, a d=0.2 would imply about 85% overlap between these populations, which makes it hard to differentiate between them. When d=0.5, the overlap shrinks to about 67%, and the difference between these populations becomes apparent, while with d=0.8, the overlap shrinks to about 53%, leading to a clear differentiation.15 In this systematic review, we used Cohen’s d to compare the effect estimates of various adherence assessment tools used in monitoring adherence as an outcome. We opted for this approach as SMD tends to overestimate the effect size, particularly in small sample size studies.
Risk of bias in individual studies
Randomised controlled trials
The quality of each randomised controlled trial (RCT) found was assessed independently by the main researcher (MA) using the Cochrane Risk of Bias Tool. The tool is selected to promote consistency in quality assessments across systematic reviews, specifically assessing the methodological risk of bias within RCTs since it has been shown to exhibit acceptable inter-rater reliability (ICC=0.58, 95% CI 0.20 to 0.81).16 The RCTs were assessed based on six risks of bias domains:
Sequence generation.
Allocation concealment.
Blinding of participants.
Incomplete outcome data.
Short-term selective outcome reporting and long-term selective outcome reporting.
Any other sources of bias.
Systematic review studies
The quality of each systematic review found was assessed independently by the main researcher (MA) using the Risk of Bias in Systematic Reviews (ROBIS) tool17 with discrepancies being resolved by author group discussion. The output assessments included three phases of, evaluating the study relevance, identifying concerns with the review process and judging the risk of bias. Phase 2 assessed four domains: the study eligibility criteria, identification and selection of studies, data collection and study appraisal and data synthesis and findings. Phase 3 includes summarising the concerns identified during the phase 2 and judging the risk of bias.
Patient and public involvement
This systematic review examined previously published literature to comprehend and convey the priorities and experiences of individuals with asthma without the direct involvement of patients or the public.
Data availability statement
No additional data are available.
Results
Study selection and characteristics
The comprehensive literature search yielded 22 414 articles identified through four databases. The study selection process is outlined in figure 1. A total of 991 duplicate articles were removed before title screening. After screening titles, 20 074 articles were excluded by title screening because the topic was not relevant to the study approach. Based on abstract screening, 1166 articles were excluded for reasons, including descriptive studies having no adherence outcomes measured, editorials, opinion papers and studies that included oral asthma or non-asthma medications. After screening abstracts, 121 articles were eligible for full-text review of which only six published articles (five systematic reviews and one RCT) were eligible for inclusion in this study narrative review synthesis.18–23 Reasons for exclusions included overlap studies appearing in included systematic review outputs, articles not related to ICS adherence (eg, diagnosis, feasibility), articles not reporting research design or methodologies, availability restricted to a conference abstract, articles only reporting study protocols, self-report studies, pharmacy refill data or no full-text paper available.
The five systematic reviews in the narrative synthesis comprised 97 studies. Most of the systematic reviews (three out of five) were performed on children with asthma, including one systematic review of children with severe asthma, while the other two included asthmatic children and adults. The included RCTs enrolled a wide age range of patients with asthma (2 to 98 years). The types of electronic technology methods included in the narrative synthesis were eHealth in two studies, digital health in one study, mHealth in three studies, and four studies evaluated EMDs. Sample sizes varied from 93 to 3913 children and 55 asthmatic adults, and 15 207 combined asthmatic children and adults published from 1998 to 2022, covering studies in the USA, Canada and the United Kingdom. The results are summarised in online supplemental table 1.
Supplemental material
Effect of the type of the electronic method
eHealth interventions
The comparison of all categories of eHealth technologies among adults and children in monitoring adherence versus control yielded a small effect in the meta-analysis study conducted by Jeminiwa et al (SMD 0.41, 95% CI 0.02 to 0.79). The level of heterogeneity between eHealth technologies in adherence results was high (I2=98%), and subgroup differences were statistically significant (χ2=8.46, df=2, p=0.01). When the adherence effects were analysed based on the type of eHealth technology used to monitor adherence, they were significant in studies using EMDs (SMD 1.19, 95% CI 0.49 to 1.89) but insignificant in those using pharmacy refill data (SMD −0.13, 95% CI −0.70–0.44) or self-reports (SMD 0.25, 95% CI −0.10–0.60). Analysis of five pooled studies among adults and children on adherence to ICS, including social media via an interactive platform, electronic health records, interactive voice response (IVR), speech recognition and telephone calls by health professionals against control, resulted in insignificant effects on adherence (SMD 0.20, 95% CI −0.02–0.43) (p=0.07).20 A narrative-systematic review conducted by Pearce et al among children with asthma included one study evaluating a web‐based interactive education and monitoring system based on social cognitive theory and eHealth theoretical models compared with receiving an asthma education manual among 42 asthmatic children. Compared with the baseline adherence rate for both groups (38%), the mean change in adherence increased by 11.2% in the intervention group to a 4.4% decrease in the control group (p=0.67).21
Electronic monitoring devices
A meta-analysis by Chan et al included seven studies and conducted analysis by the type of electronic technology among children and adults and observed statistically significant improvement in adherence in the EMD group compared with the control group with a mean difference (MD 23% higher, 95% CI 10.84 to 34.16) (p=0.0002).19 A narrative-systematic review by Pearce et al included three studies evaluating EMDs among children. Two studies compared EMDs with feedback versus EMDs alone. One study showed 70% versus 49% median adherence for the intervention group (p<0.001)24 and the second study showed 79% versus 57.9% for the intervention group (p<0.01).25 The third study compared the adherence interventions among asthmatic children with EMDs with audio‐visual enabled (intervention group) to EMDs with audio‐visual disabled (control group) every 2 months for 6 months period.26 The median adherence in the intervention group was 84% (10th/90th percentile 54%–96%), compared with 30% in the control group (10th/90th percentile 8%–68%), p<0.0001.21 A meta-analysis of 10 RCTs by Lee et al evaluated EMDs with clinical feedback compared with usual care or placebo group among 1123 asthmatic children and revealed that the EMD group was 1.50 times (RR=1.50, 95% CI 1.19 to 1.90) more likely to adhere to inhaler therapy compared with the control group (p<0.001) with medium-to-large effect size (g=0.64). However, there were no significant differences in asthma exacerbation events per year (risk ratio 0.89, 95% CI 0.45 to 1.75) (p=0.72), or asthma control using ACQ scores (Z=−0.91, p=0.36) and ACT scores (Z=0.95, p=0.34) when compared with control, but one clinical trial showed a significant improvement in children ACT scores in the intervention group than the control group (p=0.02) with a small effect size (g=0.33).22 The Boutopoulou et al’s systematic review was conducted to assess interventions on adherence to treatment in children with severe asthma and included a prospective median of 92 days observational cohort study that evaluated the adherence rate of 93 outpatient severe asthmatic children by an EMD (5–17 years old).13 The adherence rate improved from a baseline range of adherence rate from 21%–99% (median 74%) to ≥80% adherence rate for 39 patients, 60%–79% adherence rate for 25 patients (42%), and <60% adherence rate for 29 patients (31%). However, suboptimal adherence (adherence rate <80%) remained prevalent among all children with severe asthma representing 58%.18 A randomised clinical trial conducted by Berg et al compared the monitoring of adherence to any inhaled asthma medications through paper diary records and EMDs using the metered dose inhaler (MDI) Chronolog among 55 adult asthmatic patients. The MDI Chronolog records the date and time of each inhaled activation. The self-report measure used was a daily asthma paper diary. Adherence rates measured by EMDs (MDI Chronolog) showed 26% of the experimental group had >80% adherence rates versus 4% in the control group, although in each case, self-reported compliance was higher than the monitored adherence.23
mHealth (Text message services)
Four studies included in the meta-analysis conducted by Chan et al demonstrated that using a short text message service had improved adherence to therapy in children and adults with asthma compared with controls, with a mean difference (MD 12%, 95% CI 6.22 to 18.03) (p=0.0001).19 Jeminiwa et al’s quantitative analysis of the mHealth application in the form of text messages, either primarily or as an adjunct reminder and an audio-visual reminder, demonstrated overall improvements in adherence to ICS among adults and children across different methods used for adherence monitoring (SMD 0.96, 95% CI 0.28 to 1.64). The adherence improvement in studies utilising EMDs to monitor adherence was 1.28, 95% CI 0.41 to 2.14, and in those using self-reports was 0.52, 95% CI 0.23 to 0.82.20 A further narrative-systematic review among children with asthma by Pearce et al included one study on automated text message reminder interventions. The mHealth intervention group had a text message reminder, each with a tip about the value of regular controller use, compared with a control group who received only two reminders to synchronise their sensors for 30 days.
The mean adherence rates during the 30-day intervention were 34% for the intervention group and 40% for the control (p=0.56). There was also no significant difference between the intervention and control groups after adjusting for age and parental education, with none of the cases exceeding the 80% adherence threshold (control=32% vs intervention=36%, p=0.73).21
Digital interventions
The most recent systematic review and meta-analysis by Chan et al evaluated published articles up to June 2020 and assessed the effectiveness of various digital technologies among children and adult asthmatic patients. The digital intervention group showed a mean adherence percentage improvement of MD of 14.66% (95% CI 7.74 to 21.57) as compared with a control group without digital interventions. The heterogeneity of digital technologies in adherence results was high (I2=94%) (I2 value of 75% to 100% represents considerable heterogeneity).27 The various scales of asthma control among the digital interventions group showed a small improvement effect than the control group, with a 67% to 85% overlap between the two groups (SMD 0.31, 95% CI 0.17 to 0.44). There was also a small improvement in asthma-related quality of life in the digital interventions group to the control group and again demonstrated an overlap of 67%–85% between the two groups (SMD 0.26, 95% CI 0.07 to 0.45) (p=0.007).
The number of patients with ≥1 asthma exacerbation was reduced by 47% in the digital interventions group compared with the control (risk ratio 0.53, 95% CI 0.32 to 0.91) (p=0.02). However, there were no significant differences in FEV1, and there were no data on missed school or workdays, cost-effectiveness or adverse events.19
Quality assessment
Quality assessment of randomised the clinical trial
The quality assessment of the RCT was assessed using the Cochrane risk-of-bias tool.16 The findings for the risk-of-bias summary are shown in table 1. Berg et al reported an overall ‘some concerns’ bias since the measurement of the outcome could have been influenced by the knowledge of the adherence intervention received.
Risk of bias using Cochrane risk-of-bias tool
Quality assessment of the systematic reviews
The quality assessment of each included systematic review was assessed independently by the main researcher using the ROBIS tool.17 The findings for the risk-of-bias summary are shown in table 2. The majority (80%) of the systematic reviews have a low risk of bias across the four domains. Boutopoulou et al had an overall ‘high risk’ bias since, insufficient details were provided about the included studies eligibility criteria, study populations or study designs. Some risk of bias may have been introduced through the data collection or assessment processes.
Risk of bias using ROBIS tool
Discussion
Electronic methods (eHealth and digital) demonstrated benefits in monitoring and improving adherence rates to inhaled asthma medications in six published articles (five systematic reviews and one RCT) comprising 98 studies published from 1998 to 2022 in the USA, Canada and UK. Distinguishing between the electronic methods utilisation in primary and hospital care is challenging due to the diverse healthcare systems the data obtained from. Children were the primary focus of the reviews due to their inclusion in all of them, with only two covering adults and children. The broad age range of 2–98 years strengthens the generalisability of these results since no significant differences were found for the participant age range of 2–98 years for a total of 15 207 participants from 30 studies. EMDs were the most promising electronic technology demonstrating an average improvement in adherence rate of 23%, with children being 1.5 times more likely to adhere to their inhalers than non-EMD users with medium-to-large effect size (g=0.64). Adherence rates were also improved using mHealth (text message services) by an average of 12%. The effectiveness of asthma-related clinical outcomes was small, manifesting a small to medium effect for various asthma control scales (SMD=0.31) and a small effect in asthma-related quality of life (SMD 0.26) (p=0.007). There is still uncertainty regarding the effectiveness of electronic methods in reducing asthma exacerbations. There was variation in exacerbation reduction ‘between the studied interventions’ that ranged from a significant reduction of 47% (p=0.02) to a non-significant reduction of 11% (p=0.72), thus arguing for further studies to confirm or refute this effect. The effectiveness of electronic methods in improving asthma control and quality of life remains small since their evidence base is uncertain. While this systematic review brings a unique summary of systematic reviews in one place, it highlights the inconsistency and overlapping use of terminology describing electronic methods for monitoring adherence (see table 3). In this review, we found little data on the utility of electronic devices in adherence management in severe asthma and no data on the cost-effectiveness of such EMD clinical use.
Description of electronic technologies for monitoring adherence to inhaled asthma medications
EMDs showed the most promising adherence improvement than other electronic methods. EMDs record daily usage and exchange data via mobile applications and a website platform between patients with asthma and HCP, which varies from using the EMDs alone.28 This connected inhaler system (CIS), such as those of the SmartInhaler (Adherium) and Propeller Health, uses sensors connected to an inhaler device that transmits drug usage details via the Bluetooth system to an application on a patient smartphone, which in turn shares such data on a web platform that is accessible to the HCP, thus providing objective and live adherence data. The CIS (EMD+HCP feedback) achieved higher adherence rates (mean adherence 79% vs 57.9%) (p<0.01) and (median adherence 70% vs 49%) (p<0.001). Moreover, some EMDs use acoustic technologies to ascertain actual drug inhalation and inhalation technique, which may overcome dose dumping issues and provide HCP feedback on inhaler technique issues SmartInhaler (Adherium). EMDs have been combined with an asthma biomarker in the form of exhaled fractional nitric oxide (FeNO) for adherence monitoring (FeNO suppression test). This method can detect non-adherence by identifying previously non-respondents that respond well to an EMD-monitored high-dose ICS therapy, compared with non-respondents, despite the adequate level of adherence (ICS resistant) who may require alternative treatments such as escalation to biologic therapy.29 Owing to improved adherence to ICS and consequent improvement in asthma control, the FeNO suppression test led to significantly fewer patients with uncontrolled asthma progressing to biologic therapy.8 Although EMDs improve adherence, the associated costs of using EMDs with extra/fewer resources allocated by more/less GP/pharmacist/nurse visits for data collection and interpretation need to be considered.30 Considering the direct/indirect cost of adherence visits, time and the cost of the devices, affordability needs to be evaluated, using this technology in monitoring adherence. MHealth (text messages) showed adherence improvement, particularly among adolescents. This population benefited from this type of reminder system by being more proficient users of text messaging and reported the usefulness of a text messaging reminder system for asthma.31 However, it is also uncertain whether adherence improvement will remain after the patients with asthma recognise, they are not monitored. A web‐based interactive education and monitoring system by education, self‐monitoring and rewards showed an insignificant adherence effect compared with only receiving an asthma education manual (p=0.67). Moreover, studies using pharmacy refill data or self-report, electronic health records, IVR and HCP telephone calls did not show a significant adherence effect.21
The advent of electronic methods in asthma management was associated with a small improvement in asthma-related clinical outcomes and quality of life in most studies. Such observed effect may be related to the significant heterogeneity of studies and technologies used in the literature. In addition, electronic methods associated improvement in adherence may still be variable and inadequate, thus not reaching the required level to affect the necessary improvement in asthma outcomes. Inadequate adherence is common in asthma.32 An adherence rate of 80% is suggested to improve asthma control and reduce exacerbations and oral corticosteroid use.33 34 Also, other disease factors such as asthma severity or comorbidities associated with asthma may have contributed to the small observed clinical improvements. Furthermore, the variability in the adherence intervention periods among different studies that ranged from 3 weeks to 24 months, meant a significant variation in adherence rates and any consequent clinical effect.20 Although the small improvement in asthma clinical outcomes logically would be more likely to relate to improvement in adherence rates, a Hawthorne effect, where awareness of being monitored alone can lead to clinical improvement, could not be ruled out.35
Electronic methods yielded variable adherence effects ranging from small–large (eHealth (SMD 0.41, 95% CI 0.02 to 0.79)), and a wide range adherence improvement rate (digital (MD 14.66% higher, 95% CI 7.74 to 21.57)). There was also significant heterogeneity in studies reporting adherence results (eHealth I2=98%, digital I2=94%). Absence of standardisation of terminology to describe electronic methods may contribute to such variation.36 37 Significant overlap is evident among eHealth and digital health technologies in monitoring adherence since various electronic technologies fall under the umbrella of eHealth and digital health with mutually inclusive variations in the electronic technologies (see figure 2). Although eHealth includes public health monitoring cost-effectively and digital health includes using online platforms to address health needs from a consumer perspective, various technologies with variable performance that fall under eHealth and the digital umbrella require standardisation. This variability makes it challenging to classify them into specific groups and highlights the need for future research to improve classification clarity in this area. Developing a standardised definition of electronic methods for monitoring inhaled asthma medication is needed to improve comparisons between such technologies and to study their cost-effectiveness.38 39
Electronic technologies for monitoring adherence to inhaled asthma medications.
Conclusion
Electronic methods have shown a consistently positive effect on monitoring adherence to inhaled medications in patients with asthma. EMDs are the most promising effective technology among children and adults with asthma, followed by mHealth. Adherence improvement was associated with small clinical improvement and asthma-related quality of life. The absence of a uniform definition of electronic methods with the variation of electronic technologies needs to be standardised, working towards a more unified electronic method. The current gaps in the literature on using electronic methods include the heterogeneity of electronic technologies used in monitoring adherence. The absence of research data on cost-effectiveness studies focusing on severe asthma patients highlights the need for further research in this field.
Data availability statement
No data are available.
Ethics statements
Patient consent for publication
Ethics approval
Not applicable.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Contributors MA is CI-leading study design, data collection, manuscript preparation and responsible for the overall content as guarantor. JFM and AM are the first and second reviewers. JFM developed statistical expertise. AM provided clinical expertise. All authors provided feedback and contributed to the data interpretation and preparation of the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests AM declares no conflict of interest in relation to this work but has received personal and departmental funding from GSK, AZ, Teva, Chiesi, Novartis, BI for talks, advisory board, and educational grants.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.