Article Text
Abstract
Objectives This meta-analysis aims to evaluate the effect of n-3 polyunsaturated fatty acids (PUFAs) as a part of parenteral nutrition in patients undergoing liver surgery.
Design Systematic review and meta-analysis.
Data sources PubMed, the Cochrane Central Register of Controlled Trials, Springer link, Web of Science, China National Knowledge Infrastructure and VIP Database.
Eligibility criteria We included randomised controlled trials (RCTs) and evaluated the outcomes of liver function, inflammatory reaction, the influence of certain markers of the immune system, and specific clinical indexes for patients undergoing liver surgery and receiving parenteral nutrition with n-3 PUFAs.
Data extraction and synthesis The Cochrane Collaboration’s tool was used to assess the risk of bias for each study. Findings were summarised in Grades of Recommendation, Assessment, Development and Evaluation evidence profiles and synthesised qualitatively.
Results Eight RCTs, including 748 patients (trial: 374; control: 374), were included in the meta-analysis. Compared with patients in the control group, the patients in the n-3 PUFA group who underwent liver surgery had significantly lower aspartate aminotransferase (mean difference, MD −42.72 (95% CI −71.91 to –13.52); p=0.004), alanine aminotransferase (MD −38.90 (95% CI −65.44 to –12.37); p=0.004), white cell count (MD −0.93 (95% CI −1.60 to –0.26); p=0.007) and IL-6 (MD −11.37 (95% CI −14.62 to –8.13); p<0.00001) levels and a higher albumin level (MD 0.42 (95% CI 0.26 to 0.57); p<0.00001). They also had fewer infection complications (OR 0.44 (95% CI 0.28 to 0.68); p=0.0003) and a shorter duration of hospital stay (MD −2.17 (95% CI −3.04 to –1.3); p<0.00001) than the controls. However, there were no significant differences in terms of total bilirubin, TNF-α, IL-2, IgA, IgG, IgM and CD3, biliary leakage and mortality between the two groups.
Conclusions We found that n-3 PUFAs can benefit patients undergoing liver surgery by improving liver function and certain clinical indexes and decreasing related inflammation factors. However, there are limited RCTs on the application of n-3 PUFAs for patients undergoing liver surgery. Further evidence of the benefit of n-3 PUFAs in these patients warrants further exploration.
- nutrition
- nutritional support
- surgery
- hepatobiliary surgery
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
We used the Grading of Recommendation, Assessment, Development and Evaluation approach to evaluate the strength and quality of the evidence.
The quality of the included studies was mostly moderate to high.
The evaluation of postoperative outcomes of n-3 polyunsaturated fatty acids for patients not only included liver function and inflammatory reactions but also involved markers of the immune system, postoperative complications and hospital stays in this study.
The preoperative symptoms and the scope and degree of surgical resection are not considered.
Introduction
Liver cancer is the sixth most common cancer and the third-leading cause of cancer-related death.1 Based on guidelines for liver cancer treatment and management, liver surgery is the optimal method of cure for this malignancy when contraindications are excluded.2–5 Improving nutritional status is helpful for postoperative recovery in patients who undergo liver surgery. However, most patients cannot rely on enteral nutrition early after surgery when their intestinal function has not recovered. Thus, parenteral nutrition (PN) has been widely used to prevent malnutrition. On the other hand, PN may also contribute to severe hepatic complications and the inflammatory response.6 Increasing evidence has demonstrated that an excessive inflammatory response could cause severe postoperative complications in patients who undergo hepatic resection.7 8 Therefore, alleviating the inflammatory response is necessary to improve postoperative recovery and prognosis for patients who undergo liver surgery.
Currently, due to their effect on regulating lipid metabolism, decreasing oxidative stress and maintaining intestinal health, the n-3 polyunsaturated fatty acids (PUFAs) have been extensively applied as a commercial food supplement to prevent chronic disorders, including cancer and cardiovascular and metabolic diseases.9–13 As a heterogeneous mix of fatty acids, n-3 PUFAs, including long-chain α-linolenic acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), are generally derived from plants and fish.14–16 To date, EPA and DHA have been demonstrated to be the most bioactive components among them. They have been shown to be significant precursors of metabolites that act as lipid mediators.17 For example, DHA is one of the critical components of the cell membrane.18 Their intermediate, docosapentaenoic acid, may also positively affect humans.19 20
n-3 PUFAs were widely used as a component of the PN emulsion to provide energy and essential fatty acids in surgical patients, including those who undergo liver surgery.21 22 Unlike the effects of n-6 PUFAs, which are associated with the inhibition of cell-mediated immunity and enhancement of inflammation, n-3 PUFAs are considered to be immunomodulators with anti-inflammatory effects.23–25 The mechanism may be associated with the competitive inhibition of the proinflammatory property of arachidonic acids (AA) and cytokines by the conversion of EPA and DHA to prostaglandin series 3 (PGE3, PGI3 and TXA3) and leukotrienes series 5 (LTB5, LTC5 and LTD5).26 27 Therefore, various reports have shown that increasing the ratio of n-3: n-6 PUFAs in the PN emulsion on preoperative or postoperative patients undergoing liver surgery can improve prognosis.28–30
Randomised controlled trials (RCTs) have been designed to establish causal associations in clinical studies with the highest level of evidence.31 A previous systematic review reported that n-3 PUFAs have a positive effect on liver function and inflammatory reactions in patients after liver surgery.29 However, these studies have been limited by small sample size or imperfect design, and postoperative complications or hospital stays were not studied. In our meta-analysis of RCTs, we focused on the outcomes of liver function (AST, aspartate aminotransferase; ALT, alanine aminotransferase; Tbil, total bilirubin; Alb, albumin), inflammatory reaction (white cell count, WCC; TNF-α, tumour necrosis factor-alpha; IL-6, interleukin-6; IL-2, interleukin-2), the influence of certain markers of immune system (IgA, IgG, IgM, CD3, CD3-lymphocytes) and specific clinical indexes (infection, biliary leakage, duration of hospital stays and mortality) for patients undergoing liver surgery and receiving the PN with n-3 PUFAs.
Materials and methods
Overview
The process of preparation and presentation of this review was based on the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)32 33 and the Cochrane Handbook for Systematic Reviews of Intervention.34 The prospective protocol of this systematic review with meta-analysis of RCTs was represented and registered in PROSPERO with the register No CRD42022296600. The PRISMA 2020 checklist and the AMSTAR-2 were used to assess the quality of this meta-analysis.35
Literature search strategy
All RCTs published before April 2022 that explored the effect of n-3 PUFAs in patients undergoing liver surgery were included in this review. The literature was mainly retrieved from the following databases: PubMed, the Cochrane Central Register of Controlled Trials, Springer link, Web of Science, China National Knowledge Infrastructure and VIP Database. The search strategy included a combination of keywords and MeSH terms. For example, the search string on PubMed was as follows: (‘liver resection’ OR ‘hepatic resection’ OR ‘hepatectomy’ OR ‘liver surgery’ OR ‘hepatic surgery’ OR ‘liver transplantation’) AND (‘omega-3 fatty acids’ OR ‘n-3 PUFAs’ OR ‘n-3 fatty acids’ OR ‘n-3 polyunsaturated fatty acid’ OR ‘fish oil’ OR ‘alpha-linolenic acid’ OR ‘docosahexaenoic acid’ OR ‘eicosapentaenoic acid’) AND (‘randomized controlled trial’). The electronic database search was supplemented by a manual search of the reference lists of included articles. All included RCTs written in English or Chinese were identified independently by two investigators (Authors: ZH and CW) by reviewing the titles, abstracts and full texts, and discrepancies were resolved with the other investigator by consensus. The precise search strategy is shown in online supplemental material. The screening and storing process for all articles were conducted in the software EndNote V.20 version.36
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Supplemental material
Trial screening
Clinical trials that met all of the following criteria were enrolled: (1) adult (≥18 years old) patients undergoing liver surgery; (2) RCTs; (3) patients who were administered PN postoperatively for no more than 7 days (avoid long-term applied effects); (4) trial group with n-3 PUFA-enriched lipid emulsion vs control group with standard lipid emulsion or other fatty acids; (5) full-length journal articles and (6) at least one of the following postoperative outcomes: liver function, markers of inflammation, immune status, postoperative complications, duration of hospital stay and mortality. Articles were excluded when they were either unoriginal or missing relevant information. Investigators were not contacted with the authors of RCTs included in this review.
Data extraction
The data of included trials in this meta-analysis were independently collected by two coauthors (YL and AD), and any discrepancies were discussed until a consensus was reached. Collected data included the first author, year of publication, the country where the study were conducted, the total number of patients included in the study, study design, surgical methods, duration and dose of the application of n-3 PUFAs, time of blood test, covariates adjusted in each included study, postoperative liver function (AST, ALT, Tbil, Alb), postoperative markers of inflammation (WCC, TNF-α, IL-6, IL-2) and the immune system (IgA, IgG, IgM, CD3), postoperative complication (infection; biliary leakage), duration of hospital stays and mortality. The editing and storing of all data was conducted in the Microsoft V.16.61 version software.37
Risk of bias assessment
The bias risk of all included studies was assessed by the Cochrane Collaboration risk of bias tool (Cochrane RoB2 version).34 Seven methodological items based on the Cochrane Collaboration’s tool for assessing each study’s risk of bias were included following randomised sequence generation, method of concealing allocation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other potential biases. Each item was evaluated for the following three outcomes: low risk (meeting the conditions of each item), high risk (not meeting the conditions of each item) and some concerns (cannot evaluate whether meeting the conditions of each item).38 Finally, the quality of evidence was categorised as good (low risk for all items), fair (low risk for more than three items) and poor (low risk for equivalent and less than three items).39
The certainty of the evidence for the outcome was based on the guidelines of the Grades of Recommendation, Assessment, Development and Evaluation (GRADE).40 The criteria items consist of study design, limitations, inconsistency, indirectness, imprecision and other considerations. Each item included the following three levels: high (indicating that further research is unlikely to change our confidence in the estimate of the effect), moderate (indicating that further research may have a significant impact on our confidence in the assessment of the effect and may change the estimate), and low (indicating that further research may have an important impact on our confidence in the estimate of the effect and may change the estimate).
Data synthesis
This meta-analysis was conducted using Review Manager V.5.4 software.39 The pooled effect size was calculated for the postoperative outcomes of each included study containing sample size, mean, SD, SE of the mean and/or 95% CI. The mean difference (MD) standardised MD (SMD), OR, 95% CI, z value, alpha value for z, I2 (I2 for consistency) values and Q statistic for heterogeneity were calculated or extracted from Review Manager Software. The pooled weighted MD and its 95% CI were used to express continuous data when they could be converted into the same units, such as the length of hospital stay and markers of liver function, inflammation and immune system, and the SMD was used for data of different units.41 The SMD was calculated by subtracting the postintervention data from the baseline value based on the Cochrane Handbook recommendations if certain studies only reported CI or SE of the mean.42 The OR and its CI were selected to express dichotomous data (mortality rates, postoperative infection and biliary leakage). A two-tailed alpha value ≤0.05 for z and nonoverlapping 95% CI were regarded to express statistically significant changes in postoperative outcomes between the trial and control groups. A p<0.10 for the Cochran Q statistic and I2 values greater than 50% were considered significant heterogeneity. To reduce the heterogeneity across these studies, the investigators (FL and YZ) calculated the pooled effect size by using a random effect model when I2 values ≥50% or using a fixed effect model when I2 values <50%.34
Before evaluating the outcome of these RCTs, investigators (J-YS and PC) determined the prior hypotheses of heterogeneity, including surgical methods (hepatectomy or liver transplantation), intraoperative situation, brand name of n-3 PUFA, applied duration and dose, time of blood test and covariates adjusted in the statistical analysis. The I2 was less than 50%, or the corresponding p values were greater than 0.10, indicating that the heterogeneity was not significant. Subgroup analysis was carried out to explore the factors resulting in heterogeneity of the type of administration. Due to the small number of included studies of each evaluated outcome, subgroup analysis was not performed in this meta-analysis.
Patient and public involvement
No patients or members of the public were involved in the design or conduct of this study.
Results
Study identification and characteristics
A flow diagram of the study identification process is shown in figure 1. A total of 1806 relevant articles were identified by reviewing the title and abstract in the electronic study. Eight RCTs were included in this meta-analysis (figure 1) after the following exclusion criteria were applied: (1) irrelevant and duplicate studies (1782) and 3 additional studies were obtained by handsearching the references, and full texts of the remaining 27 articles were retrieved for review. (2) Thirteen studies were not linked to n-3 PUFAs and three articles were reviews.29 43 44 (3) One excluded study involved other surgeries.45 (4) One study showed the same data as another article from the same team.46 47 (5) One article lacked outcomes of interest.48
Summary of study identification and selection.
All 8 RCTs, which included a total of 748 patients (trial: 374; control: 374), were conducted in China or Egypt. Two of the RCTs were published in Chinese.49 50 In five studies, all patients underwent hepatectomy.49 51–54 In the other three studies, all participants were status post liver transplantation.47 50 55 Except for two studies by the product of Fresenius Kabi containing soy oil-medium chain triglycerides—olive oil—fish oil lipid,52 55 the remaining six studies used a product of n-3 PUFAs, Omegaven. The application duration of n-3 PUFA for liver surgery patients was 5 days in three RCTs,51 53 54 6 days in the other three RCTs,49 50 52 and 2 and 7 days in the remaining two studies.47 55 Among these included RCTs, the postoperative time of blood test was the third day in four articles,49 51 54 55 the second day in two articles,47 50 the sixth day in one article53 and unknown in the remaining one study.52 Online supplemental table 1 summarises the detailed characteristics of the included RCTs.
Assessment of bias risk
Among the eight RCTs, four trials used appropriate methods to generate the randomisation sequence,47 49 53 54 but the methods in the other studies were unclear.50–52 55 Certain studies performed concealment of allocation appropriately. For instance, sealed nontransparent envelopes were used in two studies54 55 and the pharmacist was the only person aware of the randomisation list in one study.53 Five studies discussed the blinding of patients and personnel,51–55 and all the RCTs were considered low risk regarding blinding of outcome assessors due to the objective outcomes. Drop-out was described in 1 study after randomisation, and it was regarded as low risk because the drop-out rate was less than 10%.54 Selective outcome reporting in all studies was not identified. Five studies revealed the sources of funding or had no conflicts of interest.47 50–54 The risk of bias assessment of each methodological component from the included RCTs is shown in online supplemental figure 1.
Liver function
The levels of AST were reported in 5 RCTs,47 49 51 53 55 which included 370 patients (trial group: 184, control group: 186) undergoing liver surgery. Six RCTs mentioned the levels of ALT, Tbil and Alb; these trials included 341 patients who received n-3 PUFAs and 341 patients who received control nutrition.47 49 51 53–55 Forest plots of pooled data on liver function are shown in figure 2 and online supplemental table 2. The levels of AST (MD −42.72 (95% CI −71.91 to –13.52); p=0.004) and ALT (MD −38.90 (95% CI −65.44 to –12.37); p=0.004) were significantly lower in patients who received n-3 PUFAs, and there was no significant difference in Tbil (MD −0.97 (95% CI −2.50 to 0.57); p=0.22) between the two groups. We also found that the levels of Alb (MD 0.42 (95% CI 0.26 to 0.57); p<0.00001) in these patients were significantly higher than those in the control group. However, there were some heterogeneities among the studies that provided AST (I2=84%, p≤0.0001), ALT (I2=85%, p≤0.00001) and Tbil (I2=56%, p=0.05) data.
Forest plots of pooled data on liver function. (A) AST, (B) ALT, (C) Alb, (D) Tbil. Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; IV, inverse variance; PUFAs, polyunsaturated fatty acid; Tbil, total bilirubin.
Markers of inflammation
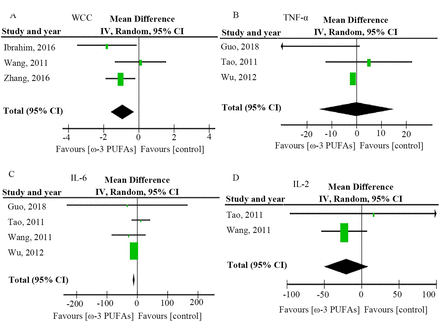
Forest plots of pooled data on markers of inflammation are shown in figure 3 and online supplemental table 2. Three RCTs showed a significantly lower trend in WCCs (MD −0.93 (95% CI −1.60 to –0.26); p=0.007) in the n-3 PUFA group compared with the control group.49 54 55 Four RCTs also described a significantly lower trend in the levels of IL-6 (MD −11.37 (95% CI −14.62 to –8.13); p<0.00001) in the n-3 PUFA group compared with the control group.49 50 52 53 However, there was no significant difference in the levels of TNF-α (MD −0.01 (95% CI −15.08 to 15.06); p=1.00) in three RCTs50 52 53 and IL-2 (MD −20.72 (95% CI −50.34 to 8.89); p=0.17) in two RCTs.49 50 Three RCTs showed certain heterogeneities in the level of TNF-α (I2=53%, p=0.12).
Forest plots of pooled data on markers of inflammation. (A) WCC, (B) TNF-α, (C) IL-6, (D) IL-2. IV, inverse variance; PUFAs, polyunsaturated fatty acids; WCC, white cell count.
Markers of the immune system
The forest plots of markers of the immune system are shown in figure 4 and online supplemental table 2. Compared with the control group, patients in the n-3 PUFA group had no significant discrepancies in the levels of IgA (MD 0.20 (95% CI −0.00 to –0.40); p=0.05) in three RCTs,47 49 52 IgG (MD −0.36 (95% CI −1.47 to 0.75); p=0.53) in two studies,47 49 IgM (MD −0.00 (95% CI −0.11 to 0.10); p=0.96) in three studies,47 49 52 and CD3 (MD 0.94 (95% CI −6.76 to 8.64); p=0.81) in two studies.49 52 Only two studies mentioned the outcome of CD3; thus, the heterogeneities (I2=87%, p=0.005) between these studies were not discussed.
Forest plots of pooled data on markers of the immune system. (A) IgA, (B) IgG, (C) IgM, (D) CD3. IV, inverse variance.
Clinical indexes
Forest plots of pooled data on clinical indexes are described in figure 5 and online supplemental table 2. Five RCTs described the outcomes of infection, duration of hospital stay and mortality of patients who received n-3 PUFAs.47 49 51 53 54 These patients had fewer infectious complications (OR 0.44 (95% CI 0.28 to 0.68); p=0.0003) and shorter hospital stays (MD −2.17 (95% CI −3.04 to –1.3); p<0.00001). Based on the analysis, there was no significant difference in mortality (OR 0.25 (95% CI 0.06 to 1.03); p=0.06) compared with the control group. In addition, two studies demonstrated that there was no statistical significance in the incidence of biliary leakage (OR 0.71 (95% CI 0.32 to 1.59); p=0.41) between the trial and control groups.51 54
Forest plots of pooled data on clinical indexes. (A) Infection, (B) biliary leakage, (C) duration of hospital stay, (D) Mortality. IV, inverse variance; PUFAs, polyunsaturated fatty acids.
Investigation of heterogeneity
The sensitivity analysis was conducted on the outcomes of the high heterogeneity mentioned above by removing one study at a time to recalculate the overall homogeneity and effect size. The results showed that the directed effect had no significant changes when any one study was excluded, supporting the stability of the association between n-3 PUFA application and the outcome of postoperative patients undergoing liver surgery.
Analysis of outcomes
The quality assessment of each outcome based on GRADE is shown in online supplemental tables 2 and 3. The factors for downgrading study outcomes in the meta-analysis were as follows: (1) at least one element for the risk of bias is considered to have limitations in design; (2) timing and duration of intervention were inconsistent; (3) low sample size (n<400) and (4) the time of follow-up was different. According to the GRADE, the quality was evaluated as ‘moderate’ in the outcomes of ALT, Tbil, Alb, WCC and specific clinical indexes (postoperative infection and biliary leakage) in this analysis. The remaining evaluated outcomes were regarded as low risk.
Discussion
This meta-analysis included eight RCTs, which were mainly completed in China, and the results indicated that n-3 PUFAs benefit patients who undergo liver surgery. The ESPEN guidelines suggested that clinicians increase the content of n-3 PUFAs in PN for postoperative patients with liver diseases. However, there are still few clinical studies regarding the effect of n-3 PUFAs on patients who undergo liver surgery.
As a critical effective component of PN, the benefits of n-3 PUFAs for postoperative patients have been reported in many studies. This systematic review and meta-analysis, for the first time, comprehensively illustrated the role of n-3 PUFAs in postoperative patients undergoing liver surgery. Provision of n-3 PUFAs can be significantly favourable in reducing ALT, AST, WCC and IL-6, increasing albumin levels, decreasing the incidence of infectious complications and shortening hospital stay. Based on the GRADE (online supplemental tables 2 and 3), the qualities of these outcomes of ALT, WCC, Alb and infectious complications were moderate and reliable. These results strengthen the evidence for applying n-3 PUFAs to postoperative patients undergoing liver surgery from enhanced recovery after surgery recommendations.56
Implementation of n-3 PUFA-based PN can be beneficial to liver function. The levels of AST and ALT are directly associated with the number of damaged liver cells.57 Alb, derived from the liver, was used to evaluate patients' liver function and nutritional state.58 59 The improvement in liver function could be attributed to the anti-inflammatory effect of n-3 PUFAs in reducing liver cell damage from the inflammatory response. However, as another indicator of evaluating liver function, the level of Tbil from this review had no significant changes in the intervention groups compared with the control group. One possible explanation is that different surgical approaches, such as hepatectomy and liver transplantation, determine the residual liver volume, which influences compensatory liver function. This may also be one of the reasons for high heterogeneity in the outcomes of AST, ALT and Tbil among the included RCTs of the review.
It is known that the innate immune system responds to pathogens and other stimuli by releasing multiple proinflammatory cytokines.60 For instance, IL-6 can activate antigen-specific immune responses and induce the release of TNF-α, which can cause significant tissue damage and even generate autoimmune conditions. Even though the levels of TNF-α and IL-2 were comparable in the two groups of this review, the levels of WcCs and IL-6 in the n-3 PUFA group were significantly lower than those in the control group. These results strengthen the evidence of the involvement of n-3 PUFAs in acute inflammatory responses.61–63 The potential mechanisms are presented as follows: (1) The LT5 series induced from EPA (n-3 PUFAs) was less proinflammatory than the LT4 series derived from AA and n-6 PUFAs. (2) As a crucial proinflammatory transcription factor, nuclear factor-kB (NF-kB) can induce the release of many proinflammatory genes encoding cytokines (such as TNF-α, IL6 and IL-2), adhesion molecules and chemokines, but its expression was inhibited in the n-3 PUFA group. (3) Resolvin D1 and protectin D1, which are lipid mediators derived from DHA, are associated with the resolution of inflammation and alleviation of tissue injury by neutrophils during inflammation.
There were no significant influences on IgA, IgG, IgM and CD3 outcomes based on the meta-analysis. Although recent studies have indicated that n-3 PUFAs have a dampening effect on B-cell activation, there is still some controversy in the field.64 For instance, EPA and DHA can increase the level of IgM by increasing the number of antibody-producing cells in mice and humans,65–67 while n-3 PUFAs do not alter B-cell production of IgA, IgG or IgD.68 The number of studies included in this review was insufficient to demonstrate the effect of n-3 PUFAs on the immune system in patients undergoing liver resection, and further investigation is needed.
Liver resection is associated with high rates of postoperative complications and mortality.69 In this review, the n-3 PUFA group showed a lower incidence of infectious complications and a shorter hospital stay than the control group. These results can be attributed to the regulation of n-3 PUFAs on various inflammatory, metabolic and immune processes.
n-3 PUFAs were expected to reduce mortality to a certain extent by improving liver function, alleviating the inflammatory response, decreasing the incidence of infectious complications and shortening the duration of hospital stay, as mentioned above. However, the mortality in the n-3 PUFA group was lower than that in the control group, without statistical significance, in this meta-analysis. This might be attributed to the limited number of trials and inconsistency in the follow-up time of mortality in different trials. Among the RCTs, the follow-up times were 1 month, 6 months and 1 year in three trials47 53 54 and not given in the other two trials.49 51
This meta-analysis had several limitations. (1) Since investigators did not adjust for multiple statistical tests, the potential for chance findings was present. (2) Due to an aggregate data meta-analysis, the potential for ecological fallacy existed.70 (3) The sources of high heterogeneity in certain outcomes could not be explained by sensitivity analysis and subgroup analysis due to the low number of RCTs included in this review. Likewise, it is not clear whether publication bias exists among the included RCTs. Funnel plots of the studies were produced to explain publication bias (not shown). However, only a small number of RCTs were included in this review, most of which contained a small sample size. Therefore, funnel plots and other measures to assess publication bias may not reflect publication bias and may be misleading, which were not conducted in the review.48 49 However, the potential factors resulting in high heterogeneity in the review were considered and listed in advance. (4) Only limited markers of outcomes were selected in this meta-analysis, especially markers of inflammatory reactions and the immune system. (5) This review did not clearly answer the question of the mechanism by which n-3 PUFAs improve postoperative recovery for patients undergoing liver resection, although several potential mechanisms have been proposed. Further study will concentrate on other markers and explore their potential mechanisms in animal models. (6) There were several confounding factors in this meta-analysis, such as location (mainly in China), surgical methods (hepatectomy or liver transplantation), intraoperative situation, the composition of lipid emulsion, the applied duration and dose, time of lab test and covariates adjusted in the statistical analysis, which may weaken the power of the conclusion. Thus, additional RCTs with higher methodological quality and larger sample sizes are required to obtain more robust conclusions.
Conclusion
In summary, the results of this systematic review and meta-analysis provided evidence that n-3 PUFAs could improve liver function, lower the inflammatory response, reduce postoperative complications and shorten the duration of hospital stay for patients undergoing liver surgery. However, n-3 PUFAs showed no significant influence on those patients’ relevant immune markers, probably due to the limited number of trials. The mechanism of the effect of n-3 PUFAs as part of PN for postoperative patients is not exactly clear, and it warrants further investigation with a larger sample size in randomised clinical trials.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Acknowledgments
We thank Dr Preyanuj Yamwong (the president of the Society of Parenteral and Enteral Nutrition of Thailand) from Siriraj Hospital, and Dr Lishi Sun from the Department of Haematology and Oncology of Brookdale University Hospital Medical Center (USA) for reviewing this manuscript and providing language assistance.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Z-WH and CW contributed equally.
Contributors Z-WH: conceptualisation; data curation; formal analysis; investigation; software; visualisation; roles/writing—original draft. CW: conceptualisation; investigation; resources; writing—review and editing. YL: data curation; formal analysis; investigation; resources; validation. AD: data curation; validation; investigation. F-BL: data curation; investigation; resources. J-YS: data curation; methodology; validation. PC: data curation; investigation; formal analysis. X-YY: data curation; validation; formal analysis. C-XW: data curation; validation; investigation. LR-H: data curation; investigation; software. B-HZ: conceptualisation; funding acquisition; methodology; project administration; resources; supervision; writing—review and editing; guarantor.
Funding This work was supported by Chen Xiao-ping Foundation for the Development of Science and Technology of Hubei Province (No. CXPJJH121001-2021004 and No. CXPJJH11900001-2019325) to B-HZ.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.