Article Text
Abstract
Objectives The aim of our observational study was to investigate the incidence, clinical characteristics and outcome of post-stroke recrudescence (PSR) in the Chinese population.
Design and setting Single-centre prospective observational study in China.
Participants A total of 1114 patients who had a suspected stroke were prospectively screened from October 2020 to February 2022.
Outcome measures The primary outcome was the proportion of patients with functional independence defined as a score of 0–2 on the modified Rankin Scale (mRS) at 3 months. Secondary outcomes were: early neurological improvement (ENI), defined as a National Institutes of Health Stroke Scale (NIHSS) score of 0 or an improvement of ≥2 points from admission at 24 hours; mortality within 3 months; stroke recurrence within 3 months and length of stay in hospital.
Results A total of 959 patients with cerebral infarction and 30 patients without an available magnetic resonance imaging (MRI) scan were excluded. Among the 125 included patients, 27 cases of PSR (2.4%), 50 cases of transient ischaemic attack (TIA) (4.5%) and 48 cases of stroke mimics (SMs) (4.3%) were identified. A higher frequency of infection at admission (22.2% vs 2%, p=0.007) was observed in patients with PSR compared with patients with TIA, and a lower proportion of functional independence at 3 months (80% vs 98%, p=0.015) was seen. Patients with TIA had a higher frequency of ENI compared with patients with PSR and SMs (98% vs 59.3%, p<0.001; 98% vs 52.1%, p<0.001). Patients with PSR exhibited a higher frequency of grade 2 Fazekas deep white matter hyperintensity compared with those with SMs (33.3% vs 8.3%, p=0.010).
Conclusions PSR is not uncommon in patients presenting with stroke symptoms and can be distinguished from TIA and SMs based on a combination of clinical features and trigger in the Chinese population. The neurological deficits of patients with PSR often resolve within several days following the resolution of the trigger.
- stroke
- stroke medicine
- adult neurology
- neurology
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request to the corresponding author.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This was a prospective observational study of the clinical features and outcomes of PSR in the Chinese population.
This study was conducted at a single centre, with a limited sample size.
Only suspected patients presenting within 72 hours of symptom onset were included, potentially introducing selection bias.
Patients who did not undergo an MRI scan were excluded, which may lead to an underestimation of the prevalence of PSR.
Introduction
Focal neurological deficits commonly occur as a sudden onset in patients suspected of having acute ischaemic stroke. The hyperacute clinical differentiation of stroke and non-stroke conditions remains a frequent challenge.1 A stroke mimic (SM)s is typically characterised as a condition not as a result of cerebral ischaemia, yet it exhibits neurological symptoms that are indistinguishable from those of a stroke, with an estimated incidence of approximately 2.8–16% in the emergency room.2–4 Differentiating ‘new focal neurological symptoms’ that occur in the presence of a previous stroke, known as post-stroke recrudescence (PSR)—a specific type of SMs—presents a unique challenge, with an estimated incidence of approximately 9–10%.5 6
Early identification of certain cases of SMs through relevant examinations, including clinical symptoms and ancillary tests, can be achieved; however, obtaining an early definitive diagnosis of PSR presents particular challenges. The presence or absence of ischaemic lesions can be differentiated by MRI; focal acute neurological deficits such as PSR, SMs, and transient ischaemic attack (TIA) cannot be distinguished, even after undergoing MRI examination. However, recent study has demonstrated a higher prevalence of chronic brain infarctions and white matter hyperintensities (WMHs) in patients with TIA and ischaemic stroke when compared with those with SMs, suggesting a predictive role of these markers for TIA and ischaemic stroke.7 We speculated that imaging markers might be valuable in differentiating PSR from SMs.
Although previous studies have focused on PSR, there is a scarcity of relevant research on imaging markers and prospective studies conducted within the Chinese population. The aim of this study was to compare the prevalence, clinical characteristics, imaging features and functional outcomes among patients with PSR, TIA and SMs in the Chinese population.
Methods
Design, setting and population
We conducted a single-centre prospective observational study to investigate the incidence, clinical characteristics and outcome of PSR in the Chinese population. Patients at the First Affiliated Hospital of Chongqing Medical University with suspected acute ischaemic stroke within 72 hours of symptom onset at our institution between October 2020 and February 2022 were included.
Patients were eligible if they have met the following criteria: (1) age ≥18 years and (2) suspected acute ischaemic stroke within 72 hours of symptom onset after admission. Patients were excluded from the study if: (1) they had no MRI after admission and (2) they had ischaemic infraction. PSR, characterised by the emergence of ‘new focal neurological symptoms’ in patients with a history of stroke, was diagnosed according to the criteria established by Topcuoglu et al.5 TIA relied on the presence of clinical symptoms characterised by temporary neurological dysfunction, absence of lesions on diffusion-weighted imaging of MRI and duration of less than 24 hours.8 9 SMs was defined as a condition characterised by neurological symptoms similar to those of stroke but not caused by cerebral ischaemia,9 and PSR was considered a separate entity from SMs.
Data collection
A Research Electronic Data Capture (REDCap) system was used for data collection,10 and the collected information was analysed anonymously. The following data from our study were extracted: demographics, clinical characteristics, medical history, medication use prior to admission, relevant laboratory results (white cell count, neutrophil blood cell count, neutrophil to lymphocyte ratio, high-sensitivity C reactive protein (hsCRP)), the level of serum sodium (hyponatraemia: serum sodium <135 mmol/L; hypernatraemia: serum sodium >145 mmol/L), the level of serum potassium (hypokalaemia: serum potassium <3.5 mmol/L; hyperkalaemia: serum potassium >5.5 mmol/L), National Institutes of Health Stroke Scale (NIHSS) score at admission and premorbid modified Rankin Scale (mRS) score, SMs categories, treatment details, complications, comorbidities, prognosis and length of stay.
Imaging
The imaging was evaluated by two experienced neurologists, who were blinded to the clinical data. The WMH was evaluated using fluid-attenuated inversion recovery (FLAIR) sequences of MRI scans, while periventricular hyperintensity (PVH) and deep white matter pathology (DWMP) were rated on Fazekas scale.11 Basal ganglia subscores were assigned as follows: a score of 0 indicates no lesions; a score of 1 indicates punctate foci; a score of 2 indicates beginning confluence of foci and a score of 3 indicates large confluent areas. For PVH subscores, a score of 0 indicates no lesions; a score of 1 indicates ‘caps’ or pencil lining; a score of 2 indicates smooth ‘halo’ and a score of 3 indicates irregular PVH extending into the deep white matter.11 The visually presented quantity scale of age-related white matter changes (ARWMCs) was used to evaluate WMH. The degree of hyperintense on FLAIR was graded on a scale of 0–3 in the following brain territories: bilateral frontal, occipital-parietal, basal ganglia, temporal and infratentorial brain. White matter lesions were graded on a scale of 0–3 as follows: 0=no lesions, 1=focal lesions, 2=beginning confluence of lesions, 3=diffuse involvement of the entire region with or without involvement of U fibres. Basal ganglia were rated on a scale of 0–3 as follows: 0=no lesions, 1=a single observed focal lesion (≥5 mm), 2=two or more focal lesions, 3=confluent lesions.12
Outcome measures
The primary outcome was the proportion of patients achieving functional independence at 3 months, defined as an mRS score of 0–2.13 Secondary outcomes were: (1) early neurological improvement (ENI) defined as NIHSS score of 0 or improvement ≥2 points within 24 hours from admission; (2) mortality within 3 months; (3) stroke recurrence within 3 months and (4) length of stay in hospital. Patients were followed up by telephone interview at 3 months after onset of symptoms by trained investigators based on a standardised interview protocol. Follow-up information collected at each follow-up included mRS, all causes of death and stroke recurrence.
Statistical analysis
Descriptive statistics were presented to describe the means with SD for variables with a normal distribution, and to describe the medians along with the IQR consisting of the 25th and 75th percentiles for variables with a non-normal distribution. The clinical characteristics of quantitative data among the three groups were analysed with the Kruskal-Wallis test, while the characteristics of qualitative data were compared with χ2 test or Fisher exact. Comparisons between two groups were performed with the Bonferroni correction. When comparing differences within groups for nominal and quantitative data, statistical significance was deemed significant when the p value was less than 0.017 (0.05/3), in order to account for the conduct of multiple statistical tests within the same variable. Since the prospective cohort study aimed to explore the prevalence, clinical characteristics, imaging features and functional outcomes among patients with PSR, TIA and SMs in the Chinese population, the sample size was not predetermined. A significance level of p<0.05 (two tailed) was considered statistically significant. Data analysis was conducted using SPSS V.26.0 software.
Patient and public involvement
None.
Results
Study participants and characteristics
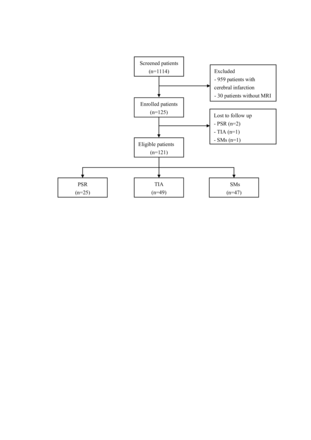
A total of 1114 patients were screened between October 2020 and February 2022. Among them, 959 patients with cerebral infarction and 30 patients without an available MRI scan were excluded. The final analysis included 125 patients, of whom, 27 individuals were diagnosed with PSR, 50 with TIA and 48 with SMs. The flow chart was illustrated in figure 1. The median age was 70 years (IQR 64–75) and 55.2% were male. The median NIHSS score on admission was 1 (IQR 0–3).
Flow chart of patient selection. PSR, post-stroke recrudescence; SMs, stroke mimics; TIA, transient ischaemic attack.
The median age of patients with PSR was 69 years (IQR 59–75), and 55.6% of them were male (table 1). Sensory disorders and dysarthria were more frequently observed in patients with PSR compared with those patients with TIA (33.3% vs 10%, p=0.027; 33.3% vs 10%, p=0.027), respectively (online supplemental table 1). The most common presenting symptoms of PSR were sensory disorders (33.3%), followed by dysarthria, hemiparesis, altered consciousness and facial paralysis. The median NIHSS score on admission was 1 (IQR 0.5–4), while the median NIHSS score at 24 hours was 1 (IQR 0–2). Additionally, 88.9% of patients with PSR had a premorbid mRS score of ≤2. Among the 27 patients with PSR, 23 had a history of infarcts, 3 had intracerebral haemorrhage, and 1 had both infarcts and intracerebral haemorrhage. The mean interval between the index stroke and recrudescence was 6.3 years (SD 7.6).
Supplemental material
Clinical characteristics in patients with PSR, TIA and SMs
Risk factors, triggers and imaging features
As shown in table 1 and the online supplemental table 1, the groups exhibited significant differences in the following aspects: ischaemic stroke (p<0.001), intracerebral haemorrhage (p=0.023), premorbid mRS ≤2 (p=0.026), infection (p=0.017) and D-dimer (p=0.002). Patients with PSR had a higher frequency of previous ischaemic stroke compared with those with TIA and SM (88.9% vs 8%, p<0.001; 88.9% vs 2.1%, p<0.001). Infection was more prevalent in patients with PSR compared with those with TIA (22.2% vs 2%; p=0.007). Moreover, although pneumonia and hsCRP were more frequent in patients with PSR, the differences did not reach statistical significance. The use of benzodiazepines and unknown time of onset of hyponatraemia did not significantly differ among the three groups. The distribution of Fazekas PVH scale and ARWMC score showed no significant differences between the groups (table 2). However, grade 2 of the Fazekas DWMP scale score was more commonly observed in patients with PSR compared with those with SM (33.3% vs 8.3%; p=0.010) in the subtype distribution of the Fazekas DWMP scale. The most common categories of SMs (online supplemental table 2) were peripheral vertigo (16.7%), peripheral neuropathy (8.3%) and cognitive decline (8.3%).
WMH and lacunes distribution in patients with PSR, TIA and SMs
Outcomes
As shown in table 3 and figure 2, the proportion of patients achieving functional independence at 3 months was lower in patients with PSR compared with patients with TIA (80% vs 98%; p=0.015). ENI was more frequently observed in patients with TIA compared with patients with PSR and SM (98% vs 59.3%, p<0.001; 98% vs 52.1%, p<0.001). There was also a higher tendency of ENI in patients with PSR compared with SMs, although the difference did not reach statistical significance. The mean length of stay was longer in patients with SMs compared with those with TIA (10.5±5.3 vs 8.2±3.6; p=0.016). Moreover, there were no significant differences among the groups in term of mortality, stroke recurrence, and the composite outcomes of mortality and stroke recurrence within 3 months.
Distribution of mRS scores at 3 months among patients with PSR, TIA and SMs. mRS, modified Rankin Scale; PSR, post-stroke recrudescence; SMs, stroke mimic; TIA, transient ischaemic attack.
Comparison of outcomes in patients with PSR, TIA and SMs
Discussion
The findings of this study indicate a significantly higher incidence of infection triggers in patients with PSR compared with those with TIA. PSR can develop following ischaemic stroke. Patients with PSR were more likely to achieve functional independence at 3 months after the resolution of the triggering event, compared with their premorbid state. ENI was more frequently observed in patients with TIA compared with those with PSR and SMs. Patients with PSR exhibited a higher incidence and extent of DWMP in Fazekas score 2 compared with those with SMs. Patients with TIA generally had shorter hospital stays compared with those with SMs.
To the best of our knowledge, this is the first prospective comparative study of PSR and SMs in the Chinese population. The prevalence rate of PSR in our study was 2.4%, which falls within the lower range of 9–10% reported in previous studies.5 6 Possible reasons for these variations include stricter MRI-based investigations in our study compared with earlier studies that relied solely on CT. Additionally, a subset of patients with PSR triggered by infections, especially those accompanied by fever, initially sought care at nearby medical facilities during COVID-19, and racial differences. Consistent with previous findings,5 our study revealed a higher likelihood of PSR among patients with a history of ischaemic stroke or intracerebral haemorrhage. Additionally, we observed that infection was a significant trigger for PSR but not for TIA. Furthermore, patients with PSR tended to exhibit higher levels of hsCRP compared with patients with TIA, although this difference did not reach statistical significance. A previous study reported a strong correlation between post-stroke infection and an elevated risk of mortality and disability.14 However, the precise underlying mechanism remains unclear. Existing evidence suggests that PSR can be induced by an immunological response triggered by infection,15 16 neurochemical mechanisms mediated by GABAA following midazolam administration17 18 and alterations in neuronal excitability and transmission due to sodium–water imbalances.5 Additionally, potential triggers for PSR or SMs include the use of benzodiazepine, elevated D-dimer levels, hyponatraemia, hypotension and exposure to stressors.5 6 19–22 This discrepancy is likely attributed to variations in enrolled patient settings, disparities in healthcare and the limited sample size of the study.
We observed recurrences of various neurological deficits, including changes in consciousness, gaze, facial paralysis, motor arm or leg, ataxia, sensory, language, dysarthria and neglect. Sensory impairments and dysarthria were the predominant neurological impairments in patients with PSR and SMs. This study is consistent with a previous study that has described functional SMs as the most common cause of SMs.4 However, a high proportion of migraine and seizure was not observed in this study, which could be attributed to factors such as differences in study population, variations in inclusion criteria and exclusion of patients with PSR. Interestingly, patients with TIA were more prone to experience ENI compared with those with PSR and SMs. Additionally, we found that neurological deficits in patients with PSR resolved within several days, and none of the patients developed intracerebral haemorrhage following tissue plasminogen activator. These findings support the view that withholding intravenous thrombolysis from patients with suspected acute stroke deficits was unwarranted, consistent with the finding reported by Topcuoglu et al.5
A previous study showed that WMH is a reliable imaging marker associated with mortality and cardiovascular risk in ischaemic stroke.23 However, the relationship between WMH markers and PSR remains unclear. Furthermore, a prior study demonstrated that the mean volume of WMH was greater in patients with TIA and minor stroke compared with SMs.24 A recent study with a sample of 2112 patients revealed significant variations in the WMH rating scale among patients with stroke, TIA and SMs.7 Our study found patients with PSR had a higher incidence of grade 2 of Fazekas DWMP according to the Fazekas scale compared with those with SMs. Therefore, we postulate that WMH could potentially serve as a marker for distinguishing patients with PSR from those with SMs. However, this hypothesis will be confirmed in prospective studies.
This study revealed that patients with PSR had a lower likelihood of achieving functional independence at 3 months compared with patients with TIA. This could be attributed to the presence of premorbid neurological deficits in patients with PSR. Furthermore, this study demonstrated that neurological function in patients with PSR was comparable with their premorbid state, indicating that symptoms can be relieved after removing the trigger. In our study, 87.2% of patients with SMs achieved functional independence at 3 months, which aligns with the findings of a report.4 Additionally, there were no significant differences observed among patients with PSR, TIA and SMs regarding stroke recurrence, mortality, or the composite outcome of mortality and stroke recurrence.
Several limitations should be taken into consideration when interpreting our results. First, we have only included suspected patients presenting within 72 hours of symptom onset which might lead to potential selection bias. Second, we excluded patients who did not underwent MRI scan which might underestimate the prevalence of PSR. Third, this is a single-centre study with a relatively small sample size; our findings needed to be validated in large-scale multicentre studies.
Conclusions
PSR is not uncommon in patients presenting with stroke symptoms and can be distinguished from TIA and SMs based on a combination of clinical features and triggers in the Chinese population. The neurological deficits of patients with PSR often resolve within several days following the resolution of the trigger.
Data availability statement
Data are available upon reasonable request. Data are available upon reasonable request to the corresponding author.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by the Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (2017-002). Informed consent was obtained from all patients or their legally authorised representatives.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
M-JP and J-LY contributed equally.
Contributors QL and M-JP—study conception and design. M-JP, J-LY, XH, LD, CC, X-NL, Z-QL and Z-JW—data collection. M-JP—writing (original draft). M-JP and J-LY—statistical analysis. All authors made the analysis and interpretation of the results. QL and PX—study supervision. QL—funding and writing (review and editing). QL accepts full responsibility for the work as guarantor and/or the conduct of the study, had access to the data and controlled the decision to publish.
Funding This study was supported by the Excellent Youth Foundation of Chongqing Scientific Committee (cstc2021jcyj-jqX0029).
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.