Article Text
Abstract
Introduction Loss of blood-brain barrier (BBB) integrity is hypothesised to be one of the earliest microvascular signs of Alzheimer’s disease (AD). Existing BBB integrity imaging methods involve contrast agents or ionising radiation, and pose limitations in terms of cost and logistics. Arterial spin labelling (ASL) perfusion MRI has been recently adapted to map the BBB permeability non-invasively. The DEveloping BBB-ASL as a non-Invasive Early biomarker (DEBBIE) consortium aims to develop this modified ASL-MRI technique for patient-specific and robust BBB permeability assessments. This article outlines the study design of the DEBBIE cohorts focused on investigating the potential of BBB-ASL as an early biomarker for AD (DEBBIE-AD).
Methods and analysis DEBBIE-AD consists of a multicohort study enrolling participants with subjective cognitive decline, mild cognitive impairment and AD, as well as age-matched healthy controls, from 13 cohorts. The precision and accuracy of BBB-ASL will be evaluated in healthy participants. The clinical value of BBB-ASL will be evaluated by comparing results with both established and novel AD biomarkers. The DEBBIE-AD study aims to provide evidence of the ability of BBB-ASL to measure BBB permeability and demonstrate its utility in AD and AD-related pathologies.
Ethics and dissemination Ethics approval was obtained for 10 cohorts, and is pending for 3 cohorts. The results of the main trial and each of the secondary endpoints will be submitted for publication in a peer-reviewed journal.
- Aging
- Magnetic resonance imaging
- Dementia
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Strengths and limitations of this study
DEveloping BBB-ASL as a non-Invasive Early biomarker-Alzheimer’s disease (DEBBIE-AD) is a large prospective observational study that uniquely focuses on testing a single promising imaging biomarker in multiple cohorts.
The outcomes of DEBBIE-AD may gain insights into the underlying mechanisms of cognitive impairment and may even present a novel imaging biomarker for disease prediction.
Although most research questions can be addressed within individual cohorts, the need for harmonisation may arise to ensure consistency and comparability.
Factors including patient motion and varying levels of atrophy may affect the acquisition and image analysis, but it is unsure yet to what extent the image quality will be degraded.
Introduction
Ageing-related cognitive impairment has emerged as one of the major public health challenges of our time, with Alzheimer’s disease (AD) being one of the primary causes.1 While the diagnosis of AD can be made based on biomarkers for amyloid-β (Aβ) plaques and τ tangles alone,2 3 other biomarkers are still needed to help unravel the complex pathological cascade of AD.4–7 Novel biomarkers may help to improve prognosis and AD subtype stratification and eventually monitor the effects of potential disease-modifying treatments.8
One of the earliest microvascular observations in the pathogenesis of AD and related dementias is the loss of integrity of the blood-brain barrier (BBB).9 10 The BBB is a cellular structure that protects the brain by regulating the transport of molecules between the blood and the interstitial fluid in the brain.11 While BBB dysfunction in AD has been recognised for some time, its importance in neurodegenerative diseases has recently been redefined as a potential biomarker implicated in vascular, inflammation and glymphatic pathways of AD pathogenesis.12 13
Encouraged by promising findings of altered albumin cerebrospinal fluid (CSF)/serum ratio in AD,14 novel BBB imaging biomarkers may uncover spatial patterns of BBB vulnerability in the brain. Compared with invasive methods to probe BBB integrity, such as positron emission tomography (PET) with radioactive isotopes and dynamic contrast-enhanced (DCE) MRI with gadolinium chelated agents, arterial spin labelling (ASL) perfusion MRI is fully non-invasive as well as cost-effective and easy to use. ASL uses magnetically labelled blood water as an endogenous tracer and can be extended to quantify BBB water exchange dynamics by separating the ASL signal into intravascular and extravascular compartments based on differences in the MRI signal characteristics of the two compartments. The BBB-ASL technique probes to quantify BBB water permeability and potentially employ it as a new biomarker of BBB dynamics.
Therefore, the DEveloping BBB-ASL as a non-Invasive Early biomarker (DEBBIE) consortium was initiated in 2020 through the Joint Programming Neurodegenerative Disease funded project ‘Novel imaging and brain stimulation methods and technologies related to neurodegenerative diseases’. Here, we describe the DEBBIE-AD study design to investigate the clinical value of BBB-ASL as an early biomarker of AD.
Objectives
Our study design is based on specific methodological and clinical research questions (RQ1A-C and RQ2A-C, respectively, as defined below).
Reproducibility (RQ1A)
Is the BBB water permeability measured with BBB-ASL reproducible in healthy subjects? The within-subject coefficient of variation of ASL cerebral blood flow (CBF) has been established to be around 10–20%, and similar reproducibility was found for the gold standard CBF acquisition technique oxygen-15-labelled water (15O-H2O) PET.15 16 Nevertheless, the reproducibility of BBB-ASL measurements still needs to be established. One pilot study17 has shown encouraging BBB-ASL reproducibility in a cohort of 10 healthy volunteers. RQ1A will investigate the reproducibility of BBB-ASL in a larger cohort of healthy volunteers (n=50). Additionally, we aim to compare the two most-used BBB-ASL acquisition techniques: multi-echo (ME) and diffusion-weighted (DW) ASL.17 18
Accuracy (RQ1B)
What is the accuracy of BBB-ASL compared with PET in measuring BBB water permeability? Currently, the measurement of blood flow with 15O-H2O-PET and 11C-butanol-PET is considered the reference standard for in vivo BBB water permeability measurements. 11C-butanol is freely diffusible through the BBB, and, in contrast, water transport is mediated by aquaporin-4 (AQP-4) channels.19 By comparing water permeability values derived by BBB-ASL with PET acquired with a simultaneous PET-MRI device, we will investigate the accuracy of our biomarker.
Normal variability (RQ1C)
What is the normal range of BBB-ASL derived values across age and sex in a cognitively healthy cohort? Haemodynamic parameters such as CBF and arterial transit time (ATT) are known to have high physiological variability across healthy volunteers.20 CBF is not only known to have short-term variability related to physiological changes such as caffeine and exercise21 but also changes significantly with age and sex.22 23 This variability may impact the BBB-ASL values in older adults. Thus, to identify abnormal patterns of BBB water permeability in pathological status, the normal variability of BBB water permeability measurements needs to be established in age and by sex.
Patients versus controls (RQ2A)
Can BBB-ASL differentiate patients with AD from healthy controls? Increased and decreased CBF patterns have already been recognised for both AD and prodromal AD stages.24 Additionally, ATT estimates have been shown to help differentiate patients with AD and controls, even when PET was already present in the model.25 For RQ2A, we will investigate if BBB water permeability differs between patients with AD and healthy controls in both regions of interest and pattern-based analysis.
BBB-ASL versus established AD biomarkers (RQ2B)
Does BBB-ASL correlate with current AD biomarkers? Recent studies have found associations between the BBB-breakdown marker CSF/blood albumin ratio and Aβ deposition,26 suggesting that BBB breakdown may play a role in amyloid-related AD pathophysiology. The accepted clinical biomarkers of AD are defined mainly by Aβ and τ pathology (neurofibrillary tangles).3 These biomarkers can also be used to stage patients across the AD pathophysiology.27 28 Furthermore, apolipoprotein E-4 (APOE-4) is established as the most prominent genetic risk factor of AD. Therefore, we will investigate associations of BBB water permeability with more established AD biomarkers: Aβ, τ, cortical atrophy and robust risk factors such as APOE-4, aiming to localise BBB-ASL alterations in the AD continuum.29
BBB-ASL in novel AD pathways (RQ2C)
Is BBB-ASL associated with vascular, inflammation and glymphatic AD markers? Cardiovascular risk factors and neuroinflammatory markers have been shown to accelerate BBB dysfunction as well as AD pathophysiology,7 30 31 and investigating small vessel disease (SVD) in relation to AD has been gaining attention.32 33 Furthermore, activated microglia as a neuroinflammatory response has been found in patients with AD,34 and BBB breakdown has been correlated with activated microglia and cognitive decline in older adults35 as well as in patients with SVD.36 Additionally, compromised functioning of the glymphatic system has been correlated with AD,37 impairing Aβ and toxin clearance. Furthermore, disturbed sleep and increased wakefulness have been found to acutely elevate Aβ production and impede Aβ clearance,38 39 concluding that unstable sleep is associated with an increased risk of AD. We will investigate the associations of cardiovascular and cerebrovascular, neuroinflammatory and sleep disturbance factors with BBB water permeability.
Background
The blood-brain barrier
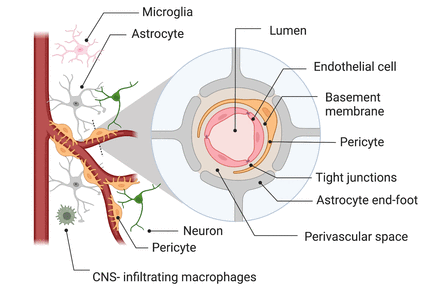
The BBB is a cellular structure that tightly regulates the transport of molecules between the blood and the central nervous system.11 At the cellular level, the BBB comprises continuous endothelial cells surrounded by pericytes, smooth muscle cells, astrocytes and microglia (figure 1), constituting the neurovascular unit. In a healthy and intact BBB, the endothelial cells are held together by tight junctions, eliminating paracellular transport across the BBB. While small lipophilic molecules, such as O2, can passively diffuse through the cell membrane, water and small ions are transported through dedicated channels regulating their exchange. AQP-4 channels are responsible for water transport and play an essential role in the glymphatic system of the brain.40
Schematic representation of the blood-brain barrier. Adapted with permission from Moyaert et al.68 CNS, central nervous system.
Measuring the permeability of the BBB to water with ASL-MRI
BBB imaging with ASL may be a viable alternative to address the aforementioned shortcomings of PET due to its non-invasiveness by using magnetically labelled blood water as an endogenous contrast agent. A detailed explanation of ASL can be found elsewhere.41 Briefly, the water of the blood in the vertebral and internal carotids is magnetically labelled. When the labelled water reaches the microvasculature, at any given time—post-labelling delay (PLD)—a fraction of the label remains in the intravascular compartment, and a fraction passes the BBB and becomes part of the interstitial fluid, that is, the extravascular compartment.42 43 Similarly to DCE, the measured MR signal can originate from the intravascular and extravascular compartments. Various characteristics of these two compartments, such as the diffusion coefficient44 and T2 time,43 can be measured by either DW- or ME-ASL, respectively, to estimate the contribution of each compartment to the total signal and the exchange time between these compartments that characterise the BBB permeability to water. If the labelled water molecules remain within the blood compartment without crossing the BBB, they will retain the diffusion and T2 relaxation properties of blood. Conversely, if these labelled water molecules cross the BBB, they will assume the properties of the grey matter. By employing various diffusion gradients (DW-ASL) or acquiring data from multiple echo times (ME-ASL), it becomes possible to characterise these two compartments and estimate the amount of water that has traversed from the blood to the brain parenchyma. Previous DW-ASL and ME-ASL studies have shown promising results for measuring this intra/extravascular compartment ratio in patients who had a stroke and animals.45–47 The water exchange time (Tex) corresponds to the average time it takes for the labelled water at the capillary bed to transit from the intravascular to extravascular space by crossing the BBB. Thus relatively high/low Tex represents a relatively low/high BBB permeability to water, respectively.
Relevance in AD
The focus on AD research is mostly based on the disease-specific molecular biomarkers amyloid (A) and τ (T) within the AT(N) framework.3 The new NIA-AA criteria for AD diagnosis consider biomarkers of cerebrovascular health (V), by incorporating MRI findings of cerebrovascular disease.7 48 49 BBB dysfunction is increasingly acknowledged as a potential early risk factor for AD,7 50 51 including increased leakage through disrupted tight junctions, decreased AQP-4 expression and reduced clearance of Aβ.52 53 BBB disruption has been correlated with Aβ burden in mice,54 and albumin levels have been found to correlate with Aβ levels.26 However, the association between BBB disruption and τ has yet to be elucidated.
Additionally, multiple novel AD pathology pathways/hypotheses (vascular, inflammatory and glymphatics mechanisms) all likely contribute to reduced BBB integrity,55 56 making this a nexus for the major pathologies involved in age-related cognitive impairment and dementia.50 55 Focal BBB breakdown, as seen in early cerebral microhaemorrhages57 58 detected with susceptibility-weighted imaging (SWI) MRI, leads to the extravasation of red blood cells to the central nervous system (CNS). Lobar microhaemorrhages are often due to cerebral amyloid angiopathy (CAA), present in many cases of mild cognitive impairment (MCI) and AD, along with Aβ deposition in the brain parenchyma.59 The BBB is involved in neuro-inflammatory processes in AD, which include microglia activation34 60 and astrocytes’ response13 61 through reactive gliosis, consequently upregulating glial fibrillar acidic protein (GFAP) and other inflammatory mediators.
Finally, studies have highlighted the role of the brain’s glymphatic system in AD by contributing to Aβ clearance.37 62 AQP-4 water channels support the function of the glymphatic system,40 and their dysfunction can negatively affect Aβ clearance.63 Recent findings also described that genetic variations of AQP-4 can negatively impact sleep quality and clearance of Aβ,50 contributing to the development of AD.64 Therefore, investigating the permeability to water of the BBB and its relation to AD biomarkers could be of significant importance as an early-stage vascular biomarker of AD and related dementias.
Current BBB biomarkers
A widely used fluid BBB biomarker is the CSF/plasma albumin ratio, as albumin is a protein naturally present in the blood but not the CSF. Thus, the presence of albumin in the CSF is used to measure BBB breakdown.65 This technique’s limitations include the absence of spatial information and its invasiveness—as it requires a lumbar puncture. Plasma BBB biomarkers include the platelet-derived growth factor β (PDGFRβ; related to neuroinflammation) and GFAP (related to astrocytosis). Injured pericytes in the neurovascular unit release PDGFRβ into the CSF and CSF PDGFRβ has been recently correlated with worse BBB integrity measured by the CSF/plasma albumin ratio,66 concluding that it may be involved in age-related BBB disruption together with neuroinflammation. GFAP is a marker of reactive astrocytes—considered critical glial cells in the support of vital CNS functions—and has been found to be significantly increased in all Aβ-positive groups compared with participants without Aβ pathology.67
Several imaging markers exist to study BBB function; a more detailed overview can be found elsewhere.68 One of the most commonly used methods is gadolinium-based contrast-enhanced MRI. Gadolinium chelates cannot cross the intact BBB due to their relatively large molecular size69 and will only leak paracellularly through disrupted tight junctions. Gadolinium alters local magnetic properties, which can be measured over time with DCE. However, gadolinium’s relatively large molecular size makes DCE-MRI less suited for measuring subtle BBB breakdown. Moreover, concerns about patient safety, comfort and environmental hazards70 71 make DCE unsuitable for repeated measurements.
An alternative approach is to measure the BBB permeability to water. One way to measure BBB permeability to water is with PET imaging combining oxygen-15-labelled water (15O-H2O) and 11C-butanol isotopes.72–74 As alcohol, 11C-butanol is a freely diffusible tracer, whereas 15O-H2O’s transport is typically limited to AQP-4 channels. Therefore, the ratio of 15O-H2O to 11C-butanol transport-based PET measurements yields an index of BBB function. However, this approach is impractical for widespread use due to its costs, invasiveness, radiation burden and logistical demands.
Methods and analysis
DEBBIE consortium
The collaborators of the DEBBIE consortium are shown in figure 2. DEBBIE builds on existing successful collaborations between consortium members and external collaborators on several international projects involving (1) dementia imaging using ASL (ASL-European Cooperation in Science and Technology (ASL-COST) – action BM113: ASL in dementia, in which ASL sequence standards and multisite reproducibility of ASL were established,75 (2) automatic processing and interpretation of ASL-CBF (through the ExploreASL76 initiative, and Eurostars ‘ASPIRE’ project, http://aspire-mri.eu/) and (3) efforts to develop best practices for perfusion MRI image processing to accelerate ASL clinical integration77 (the International Society for Magnetic Resonance in Medicine (ISMRM) – Open Science Initiative for Perfusion Imaging (OSIPI), http://osipi.ismrm.org/).
A geographical overview of the DEBBIE (DEveloping BBB-ASL as non-Invasive Early biomarker of Alzheimer’s Disease) consortium partners (orange box) and external collaborators (violet box). Franhofer MEVIS, Fraunhofer-Institut für Digitale Medizin (Fraunhofer MEVIS);LCBC, Centre for Lifespan Changes in Brain and Cognition.
BBB-ASL acquisition
ME-ASL probes the T2 relaxation time of labelled water at different inflow times (TI). As the T2 differs between blood and the extravascular compartment at 3T, it is possible to perform BBB water permeability analysis.78 79 The ME acquisition allows the assessment of the transversal relaxation time T2, which is longer for blood than for tissue. Since the ASL images with and without labelling are subtracted, all intravascular and extravascular signal is removed except for the signal coming from the labelled water molecules. If these molecules still reside in the intravascular compartment at the moment of acquisition, the measured signal will have a long T2. The more these molecules have passed the BBB into the extravascular compartment, the larger part of the measured signal will have a shorter T2.17
DEBBIE sequence
Our proposed ME-ASL uses time-encoded pseudo-continuous ASL acquisitions42 80 81 with Walsh-ordered Hadamard (HAD)-encoding and an ME segmented 3D-GRASE readout.82 Two protocols with different sub-bolus durations (SBD) are used,17 where TI=SBD+PLD (online supplemental table 1). First, the single-echo HAD8 includes seven PLDs optimised for ATT and CBF quantification, and second, the ME HAD4, includes three PLDs optimised for BBB Tex quantification. The sequence was also implemented in a vendor-independent MRI sequence development framework gammaSTAR.83 84 An illustration of perfusion-weighted images from single-TE HAD8 and ME HAD4 acquisitions is shown in online supplemental figure 1.
Supplemental material
ME-ASL image processing
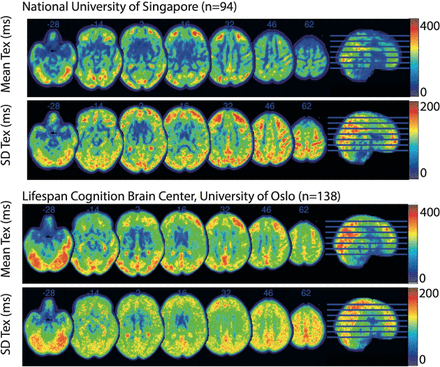
To harmonise the image processing, DEBBIE-AD uses ExploreASL.41 76 CBF, ATT and Tex quantification are performed with FSL-FABBER,76 85 implemented as a plug-in in ExploreASL. An example of the mean and SD Tex maps of two DEBBIE cohorts is shown in figure 3 to illustrate the similarities of the Tex patterns from two cohorts of similar-aged healthy adults from different sites.
Scanner-average time of exchange (Tex) maps for the two populations—National University of Singapore mean age of 56.7±6.1 with 62% women and Centre for Lifespan Changes in Brain and Cognition mean age of 52.9±15.3 with 64% women—with mean Tex and the voxel-wise between-subject SD in Montreal Neurological Institute (MNI) space.
Study participants
DEBBIE-AD includes cohorts with healthy as well as participants with cognitive impairment (table 1). The inclusion criteria for cognitively normal subjects are a global Clinical Dementia Rating (CDR) score of 0 or a score ≥27 points on the Mini-Mental State Examination (MMSE)86 or equivalent on other similar tests. For defining subjects with MCI, a global CDR score ≥0.5 point (s) or an MMSE score of 23–26 points (inclusive) or equivalent will be used. As indicated in online supplemental table 2, these criteria vary slightly between cohorts. For AD, a global CDR score of ≥2 points or an MMSE score of <23 points will be used, as well as clinical consensus. Some cohorts (online supplemental table 2) also use Montreal Cognitive Assessment (MoCA) scores for diagnosis (at least 26 for healthy controls, 18–26 for MCI and 12–26 for AD). Exclusion criteria differ between cohorts but generally include major brain lesions or psychiatric disorders, the inability to undergo all study procedures, visual or hearing impairment that would impair neuropsychological testing, severe depression (eg, Geriatric Depression Scale score ≥11 points),87 other comorbidities or medication that could impair cognition at the discretion of the cohort investigator (eg, stroke, epilepsy or use of lithium carbonate) and contraindications to MRI scanning (eg, pacemaker/defibrillator, ferromagnetic metal implants).
Cohort demographics of DEBBIE-AD
Patient and public involvement
The patients were not involved in the design of the study.
Biomarkers
The biomarkers from the DEBBIE-AD cohorts include MRI and PET (table 2) as well as blood and CSF biomarkers (table 2) and neuropsychological assessments (online supplemental table 3). In addition to BBB-ASL, the core MRI scan types (T1w, T2w, fluid-attenuated inversion recovery and diffusion-weighted imaging) are conducted in all DEBBIE-AD participants. Other advanced MRI acquisitions differ between cohorts. This may include one or more of the following types of acquisitions: three-dimensional (3D) SWI or 3D-T2*-weighting, diffusion tensor imaging, resting-state and task-based functional MRI and quantitative susceptibility mapping. Five cohorts will also acquire amyloid-PET and τ-PET scans (RQ2A-C), and one cohort will acquire neuroinflammation PET scans (RQ2C). Eight cohorts include blood sample measurements (table 2) to allow APOE genotyping, inflammatory markers (RQ2C) such as C reactive protein, astrocyte neuroinflammation marker GFAP, microglial marker triggering receptor expressed on myeloid cells 2 (TREM2) and neurodegenerative marker neurofilament light chain. Plasma biomarkers (RQ2A-B) include Aβ peptides 1–40 and 1–42, as well as total-τ and phosphorylated-τ species. Additional routine blood analysis values (eg, lipids and glucose), vitamin status (B12 and folic acid) and albumin will also be obtained in two cohorts (table 2) to investigate their potential effect on the normal BBB permeability (RQ1B). The neuropsychological assessment for each cohort includes tests on several cognitive domains (table 2).
Neuroimaging and fluid biomarkers
Analysis plan
The DEBBIE cohorts are designated a specific technical or clinical task to answer the research questions (table 1). The DEBBIE data collection has started in 2023 and is planned to finish by May 2025.
The reproducibility of BBB-ASL (RQ1A) will be investigated with healthy participants from the Ghent cohort. Good reproducibility of ME-ASL data was already shown in a pilot study of 10 participants.17 We will extend this number to provide a more robust estimation of reproducibility by including 50 healthy controls for test–retest analysis. Additionally, the Dementia Prevention Research Clinic (DPRC) cohort aims to compare DW-ASL18 with ME-ASL measurements in 40 healthy controls to investigate if the two ASL techniques used to measure BBB water permeability provide similar information.
The accuracy of BBB-ASL values (RQ1B) will be evaluated by comparing ME-ASL with 15O-H2O-PET values in a subset of 10 pigs and 12 AD, 12 MCI patients and 12 age-matched controls from the Lawson Health Research Institute (LHRI) cohort, scanned with the same protocol and same scanner. Previous studies were able to establish the accuracy of ASL in comparison with 15O-H2O-PET with 14 subjects,88 and 15O-H2O-PET has been used before to study the relation between water dynamics and early accumulation of Aβ.89
The normal variability of the BBB-ASL values (RQ1C) over age and sex will be investigated in 150 healthy participants from the Centre for Lifespan Changes in Brain and Cognition (LCBC) cohort to create an atlas of a healthy BBB across age groups and to investigate the ability of Tex to predict age above and beyond CBF and ATT.90 Previous studies examining the age and sex-related variability of CBF in healthy adults achieved significant and reliable results when including between 50 and 100 participants.91 92
To investigate if BBB-ASL can differentiate patients with AD from healthy controls (RQ2A), BBB-ASL differences between 25 patients with AD, 40 MCI patients and 40 healthy controls will be investigated with data from the DPRC cohort. We will compare global and regional Tex values between the three groups and perform analysis to determine if spatial patterns of Tex distinguish the groups. We will test if the between-group results from the DPRC cohort are consistent with other cohorts in the consortium for the same groups, namely 55 MCI and 20 patients with AD from the Amsterdam cohorts (Verloren Verbindingen Vinden (VVI) and Imaging inflammation in Alzheimer’s disease (InflammAD)), as well as 20 MCI and 20 patients with AD from the Technical University Dresden (UKD) cohort. Additionally, MRI and blood biomarkers data will be included from Centre Of Geriatrics Amsterdam (COGA) participants, which include from subjective cognitive decline (SCD) to patients with AD.
To investigate how BBB water permeability is related to the established AD biomarkers (RQ2B), we will compare the BBB-ASL values with established AD biomarkers, such as amyloid and τ, using CSF/blood, MRI and PET. The LCBC and DPRC cohorts will investigate Tex patterns with amyloid PET in subjects older than 50, for LCBC and in both 40 MCI and 20 patients with AD, for DPRC; the ‘Imaging inflammation in AD’ (InflammAD) cohort, will use blood and CSF biomarkers of amyloid and τ from 20 patients with AD; and the ‘Synaptic density and tau pathology in AD’ (SYNAPSE) cohort will include amyloid- and τ from PET, CSF and blood, from 20 Aβ-positive individuals.
Finally, to study how the BBB is implicated in (1) vascular, (2) inflammation and (3) glymphatic AD pathways (RQ2C), we will include: (a) 200 SCD/MCI participants will be selected with quite extensive white matter hyperintensities, from the NEUROlogical biomarkers of Blood, MRI and Cognition (NEURO-BMC) cohort, as well as 50 participants from the Vascular Phenotypes in a Geriatric Population (VARIATION) study which will be used to look at other vascular biomarkers including, for example, cerebral small vessel disease, microvascular function, arterial stiffness and intima-media thickness. Additionally, the Dementia Disease Initiation (DDI) cohort will focus on the differences in BBB-ASL values between Aβ+ and Aβ− groups, to identify CAA in early stages; (b) 20 MCI and 20 patients with AD from UKD will be used to focus on investigating macrostructural and microstructural patterns of sleep in relation to BBB-ASL values in participants with and without sleep disorders; and (c) 55 MCI subjects from the VVI (‘Finding Lost Connections’) cohort, 20 Aβ+ subjects from the SYNAPSE group, as well as 20 healthy controls and 20 patient with AD from the InflammAD cohorts (table 1) will be used to examine novel inflammation biomarkers (GFAP, TREM2) and a new PET tracer for activated microglia (11C-SMW139 and UCBJ).
Regional analysis
We will investigate if there is a regional variability in Tex values related to tissue type (gray matter vs white matter) or vascular territory (Anterior, middle and posterior cerebral arteries, proximal vs distal). We hypothesise that BBB disruption patterns may follow amyloid and τ accumulation patterns seen in various stages of AD pathology.93 94 Therefore, for the clinical RQs (RQ2A-C), we will focus on the regional variability of BBB-ASL values in AD—including the precuneus, cingulate cortex and orbital frontal gyrus94—as well as τ signature regions—transentorhinal, temporal and limbic regions such as the hippocampus.93
Data harmonisation
Although most of our research questions can be answered using single cohort data, we may need to combine cohorts for more statistical power for post hoc analyses (‘Analysis plan’ section). In the case of data pooling, we will attempt several harmonisation steps.
While the BBB-ASL acquisition protocols are consistent across cohorts, the other imaging techniques may vary. To harmonise the data structure, we will convert all DEBBIE-AD data to Brain Imaging Data Structure (BIDS) format95 and investigate the metadata variability between data sets (eg, due to scanner hardware or software differences and updates). The structural and ASL image data will be analysed with one pipeline (ExploreASL)76 in a standardised setting, including quantification with BASIL and FSL-FABBER.85 We will perform harmonisation on MRI images by scaling the scanner-average mean and between-subject SD maps to be the same. Our amyloid-PET data will be harmonised using the centiloid method.96
Fluid biomarkers may also vary, and harmonisation strategies should be taken into account. CSF amyloid is often harmonised using, for example, the Aβ1–42/Aβ1–40 ratio compared with Aβ1–42 alone for improved concordance, since the division by Aβ1–40 is hypothesised to correct for interindividual biological variation in amyloid production and/or clearance. Recent literature has introduced standardised cut-offs for fluid biomarkers, highlighting all these aspects.97 98
While several cohorts have specific neuropsychological tests depending on their distinct aims, most/all studies have included the same MMSE and MoCA test.
Finally, the tabular derivatives can be harmonised in the statistics using neuroCombat.99
Expected impact
The DEBBIE-AD study aims to explore the potential use of BBB disruption as a novel early biomarker for AD. This project holds promise for various applications: (1) Research advancements: by gaining a deeper understanding of the role of BBB dysfunction in AD, this study can contribute to significant advances in AD research. Even if a plasma biomarker would be an easier/more cost-effective or otherwise desirable biomarker than BBB-ASL, this would provide knowledge about such biomarkers; (2) clinical trials require more and more biomarkers for the increasing heterogeneity in AD cases and their potential response to disease-modifying treatment. The BBB being implicated in many AD pathways makes it a potentially helpful biomarker for selecting patients in clinical trials or as a secondary endpoint; (3) personalised diagnosis and treatment: in the future, BBB permeability assessment could assist in prognosis and tailoring AD medication.
Our study includes six distinct research questions. We anticipate obtaining over 1000 BBB-ASL scans from participants from various groups. Specifically, we expect to include more than 500 healthy controls, over 250 individuals with MCI, approximately 100 patients with AD and the remaining participants representing Aβ+ and SCD cohorts. The research questions described here will be addressed without pooling data. Additionally, the strength of the DEBBIE consortium is that we have the opportunity to pool data, creating an extensive data set that enables comprehensive investigations into the value of BBB-ASL.
The focus of DEBBIE-AD is to investigate the impact of this potential biomarker across different cohorts, answering specific research questions. This aspect sets DEBBIE-AD apart as all cohorts within the consortium will measure the same biomarker to explore various aspects of AD. Furthermore, we ensure consistency by using the same sequence on the same scanner vendor, using gammaSTAR technology to ensure optimised uniformity across the consortium.
Although ME ASL has been proposed previously,43 the significance of this work lies in its development of a time-efficient MRI sequence suitable for acquiring CBF, ATT and Tex in the clinical setting. To our knowledge, DEBBIE-AD is the first study protocol for evaluating a single promising MRI biomarker of AD across multiple cohorts.
This study also has some limitations. The main potential limitation of BBB-ASL as an early dementia biomarker is its methodological and physiological reliability. BBB-ASL has only been tested in healthy volunteers and not yet in patients. The inherently low signal-to-noise ratio (SNR) of the method may make the detection of clinically meaningful changes challenging. We will regularly perform quality control to assess the image quality, test preliminary associations and adapt the BBB-ASL sequence if required (eg, increasing the number of averages to boost SNR). Furthermore, BBB-ASL uses water as an endogenous tracer that has been shown to detect subtler—and potentially earlier—BBB damage than tracers with larger molecular sizes. The downside could be that BBB-ASL has a higher physiological variability, requiring larger cohorts to achieve adequate statistical power. Additionally, patient motion, atrophy and haematocrit levels may affect the acquisition and image analysis. These factors can be critical as they vary between patients and controls—for example, patients typically move more—while haematocrit and atrophy can be both methodological and physiological confounders. We will attempt several options to tackle these challenges. To optimise the quality of quantification, we intend to incorporate a T2 map for each participant based on the control images, aiming to minimise errors associated with the assumption of standard T2 relaxation values. Also, we will investigate if the image quality of the CBF, ATT and Tex maps is related to (patho-)physiological parameters such as age, sex and AD staging. Our objective is to determine whether the quality of these maps varies with age and then compare CBF quantification across a reduced number of PLDs within the same subject group, investigating potential challenges posed by ATT for BBB Tex quantification in elderly subjects. Additionally, our analysis will include testing the reliability of motion correction, specifically for the joint analysis of HAD4 and HAD8 sequences.
In summary, the mission of DEBBIE is to establish a non-invasive biomarker related to BBB health in AD. This requires demonstrating the significance of the biomarker within the disease context by comprehensive investigations of repeatability, reproducibility, variability and associations with other disease markers. The successful validation of this biomarker could have far-reaching implications, including a better understanding of the underlying mechanisms of AD and lead to earlier detection and a more accurate diagnosis.
Ethics and dissemination
The Amsterdam Medical Ethics Review Committee approved the COGA, VARIATION, VVI, SYNAPSE and InflammAD studies, in accordance with the ethical conduct and juridical laws of the Declaration of Helsinki 64th WMA General Assembly, Fortaleza, Brazil, October 2013, (www.wma.net), and in accordance with the Medical Research Involving Human Subjects Act (WMO). For the Oslo cohorts (DDI and LCBC), the regional medical research ethics committee approved the study. All further study conduct was in line with the guidelines provided by the Helsinki declaration of 1964 (revised 2013) and the Norwegian Health and Research Act. For both the Ghent and UKD cohorts, study protocol amendments are pending and being prepared, respectively, for implementing the BBB-ASL sequence in the MRI protocols. For the LHRI cohort, the animal study will be conducted according to the regulations of the Canadian Council on Animal Care and was approved by the Animal Care Committee at Western University, and the human study will be conducted in accordance with the Declaration of Helsinki ethical standards and was approved by the University Research Ethics Board. DPRC study procedures were approved by appropriate ethical review boards (University of Auckland Human Participants Ethics Committee ref 020737 and The Health and Disability Ethics Committee ref 15/NTB/202). All participants provided informed written consent before taking part in accordance with the New Zealand National Ethical Standards. The National University of Singapore Institutional Review Board approved the study in Singapore for NEURO-BMC (NUS-IRB Reference Code: NUS-IRB-2021–531). All in accordance with the Human Biomedical Research Act and the applicable laws and regulations of Singapore. All study participants provided written informed consent. On each of the research questions, separate manuscripts will be written and submitted for publication in peer-reviewed journals.
Ethics statements
Patient consent for publication
Acknowledgments
The DEBBIE committee thank all the participants of the study and the staff, nurses and technicians that make it possible.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @HMutsaerts
Contributors The DEBBIE consortium was initiated mainly by MG, KE, TF, UA, EA, JP, and HJMMM, with SH, CM, EO-I, DLT as external partners. BP, AM, MT, CM, DLT, KE, JP, HJMMM designed the study. MAB, DCH, SK, JH, and MG are responsible for the development and optimisation of the sequence for clinical practice. BP, MHS, PM, OG, JPAK, SB, WN, JW, RR, DdL, HG, LT, EEC, EO-I, JL, MB, EMvdG, MM, AF, KW, AB, LP, PS, PC, EA, UA, SH, TF, CM, MG, HJMMM are involved in the running of the study in their respective institutes. BP, AM, MT, MHS, CM, DLT, JP, MG, HJMMM drafted the manuscript. BP, AM, MT, PM, YDZ, EMvdG, FB, KE, CM, DLT, JP, MG, HJMMM, helped conceptualising and structuring the manuscript. All authors contributed information from their respective cohorts and critically revised the manuscript for content important for their respective study and cohorts. All authors approved the final version of this manuscript.
Funding The DEBBIE project (DEveloping a non-invasive Biomarker for early BBB breakdown in Alzheimer’s diseasE) has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No. 825664. It is supported through the following funding organisations under the aegis of the EU Joint Program for Neurodegenerative Disease Research (JPND2020-568-106) – FWO in Belgium, Canadian Institutes of Health Research (CIHR) in Canada, BMBF (01ED2107) in Germany, the Research Council of Norway, the Netherlands Organisation for health Research and Development and Alzheimer Nederland in The Netherlands, The Scientific and Technological Research Council of Turkey (TUBITAK; #121N030) in Turkey. The NEURO-BMC study is supported by the National Medical Research Council, Transition Award (A-0006310-00-00), Singapore. YDZ is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award TL1 TR001861. HG is supported by the Research Council of Norway (#325415). EO-I is supported by TUBITAK (#121N030). BMT is supported by ZonMW VIDI #091501719100. EMvdG is supported by a Biomedical Research grant from Alzheimer Nederland (WE.03-2021-04), and ZonMw (#10510032120006) for Mechanisms of Dementia (MODEM) as part of Onderzoeksprogramma Dementie, which is part of the Dutch National Dementia Strategy. UA is supported by CIHR — Institute of Aging and Joint Programme on Neurodegenerative Disease Research (JPND) Grant (CHIR #173743). FB and DLT are supported by the NIHR Biomedical Research Centre at UCLH. The Dementia Prevention Research Clinic is supported by the New Zealand Dementia Prevention Trust. Funding for MRI and blood biomarkers related to the study of the BBB is funded by Brain Research New Zealand and the Freemasons Foundation New Zealand. HJMMM is supported by the Dutch Heart Foundation (03-004-2020-T049) and by the Eurostars-2 joint programme with co-funding from the European Union Horizon 2020 research and innovation programme (ASPIRE E!113701), provided by the Netherlands Enterprise Agency (RvO), and by the EU Joint Program for Neurodegenerative Disease Research, provided by the Netherlands Organisation for health Research and Development and Alzheimer Nederland (DEBBIE JPND2020-568-106).
Map disclaimer The inclusion of any map (including the depiction of any boundaries therein), or of any geographical or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests FB is a consultant for Roche, Celltrion, Rewind Therapeutics, Merck, IXICO, Jansen, Combinostics, and has research agreements with Merck, Biogen, GE Healthcare, Roche. All other authors report no disclosures.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.