Article Text
Abstract
Introduction Gestational diabetes mellitus and overweight are associated with an increased likelihood of complications during birth and for the newborn baby. These complications lead to increased immediate and long-term healthcare costs as well as reduced health and well-being in women and infants. This protocol presents the health economic evaluation to investigate the cost-effectiveness of Bump2Baby and Me (B2B&Me), which is a health coaching intervention delivered via smartphone to women at risk of gestational diabetes.
Methods and analysis Using data from the B2B&Me randomised controlled trial, this economic evaluation compares costs and health effects between the intervention and control group as an incremental cost-effectiveness ratio. Direct healthcare costs, costs of pharmaceuticals and intervention costs will be included in the analysis, body weight and quality-adjusted life-years for the mother will serve as the effect outcomes. To investigate the long-term cost-effectiveness of the trial, a Markov model will be employed. Deterministic and probabilistic sensitivity analysis will be employed.
Ethics and dissemination The National Maternity Hospital Human Research and Ethics Committee was the primary approval site (EC18.2020) with approvals from University College Dublin HREC-Sciences (LS-E-20-150-OReilly), Junta de Andalucia CEIM/CEI Provincial de Granada (2087-M1-22), Monash Health HREC (RES-20-0000-892A) and National Health Service Health Research Authority and Health and Care Research Wales (HCRW) (21/WA/0022). The results from the analysis will be disseminated in scientific papers, through conference presentations and through different channels for communication within the project.
Trial registration number ACTRN12620001240932.
- HEALTH ECONOMICS
- Diabetes in pregnancy
- Randomized Controlled Trial
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Well-founded health economic methods will be used to evaluate both the short-term and long-term cost-effectiveness of the intervention.
This is a health economic analysis that covers several countries with different healthcare systems which is both a strength and a limitation.
Information on postantenatal healthcare use is self-reported.
Indirect costs (productivity losses) will not be collected during the trial.
Introduction
Maternity care is a high cost and high volume form of healthcare. Previous studies noted the need to promote high-value maternity care to improve health outcomes while curtailing escalating costs.1–4 The changing demographics of women seeking maternity care add complexity with increasing age, body mass index (BMI) and medical conditions.5 6 Gestational diabetes mellitus (GDM) is a condition of concern, with a global prevalence of between 5% and 18%.7 8
GDM is associated with an increased likelihood of pregnancy complications including pre-eclampsia, infection, obstructed labour, caesarean birth and postpartum haemorrhage.9–12 GDM also increases long-term chronic condition risk for type 2 diabetes and hypertension as well as other complications related to cardiovascular diseases.13–16 Preterm birth and special or intensive care nursery admission increases for infants born to women with GDM.12 Additionally, overweight and obesity during pregnancy lead to a higher risk of complications during birth as well as a higher risk of adverse maternal and infant outcomes.17 18 All these outcomes reduce overall short-term and long-term health and well-being of women and infants, they also come at sizeable costs to health funders.12 19 20 Due to the increased risk for type 2 diabetes and cardiovascular diseases because of GDM, the short-term and long-term direct healthcare costs as well as indirect costs are expected to be substantial.21 22 As such the GDM prevention potentially offers an opportunity to improve maternity care value—provided the proposed interventions are cost-effective.
Economic evaluation is a well-founded and widely used method within health economics where the costs and outcomes of a specific intervention are compared with an alternative.23 Cost-effectiveness (CE) analysis, which is a form of economic evaluation, procures an incremental cost-effectiveness ratio (ICER) that identifies the additional outcomes that are produced by an intervention and the additional costs of achieving them. This ratio can then be compared with established CE thresholds and to similar interventions to evaluate the CE and comparative effectiveness of the studied intervention.
The aim of the planned CE analysis described in this protocol is to evaluate the costs and health outcomes of the Bump2Baby and Me (B2B&Me) intervention compared with usual care. The results from the analysis will serve as a foundation for healthcare policy-makers in the decision-making surrounding the implementation of the B2B&Me intervention in healthcare practice for pregnant women1 in risk of gestational diabetes. More specifically, the analysis will inform about the value of the intervention within the publicly funded health and social care systems across Ireland, the UK, Spain and Australia.
Study design
A CE analysis of the B2B&Me intervention compared with usual care will be conducted. The reporting of the economic evaluation, as described in this protocol, follows the recommendations of the International Society for Pharmacoeconomics and Outcomes Research Randomised Controlled Trial Cost-Effectiveness Analysis (ISPOR RCT-CEA) Task Force24 and ISPOR Consolidated Health Economic Evaluation Reporting Standards.25 25
Comparison groups
Intervention
The intervention consists of mHealth coaching support delivered through a smartphone application from the time of randomisation up until 1 year post partum. Recruitment of participants started February 2021 and final data collection and cleaning of data is planned to be finished June 2024. Trained health coaches with a healthcare professional background including nutrition, nursing, physiotherapy or health psychology will deliver the mHealth coaching to participants and be responsible for the woman’s engagement with the app content. MHealth coaches will be trained by Liva Healthcare staff (the app developer), with support from key study personnel. Standard Liva mHealth coach training takes a four-pronged approach, inclusive of:
Patient communication; including full training on the Liva coach dashboard and patient app interface.
Behaviour change skills (BCS): coaches are recruited with existing coaching skills; this refresher course aims to build on this in line with the specific BCSs used throughout Liva coaching structures.
Practicalities; including HR topics, remote team support and communications, any programme-specific topics.
Patient safety and clinical pathways; medical and mental health governance structures and escalation pathways, any intervention-specific requirements.
In addition to this coaching training, the mHealth coaches will receive specific training related to the prevention and management of GDM, identification of and support for women with postnatal depression, breast feeding and infant feeding, infant development, and managing health for diabetes prevention. This training will be provided through an online learning platform and online delivered training by CIs SO’R, TCS and a midwife, who will be appointed as one of the mHealth coaches by Liva Healthcare.
The coaching support will be delivered through the application and will use both synchronous and asynchronous video and text messages including automated reminder and motivational messaging as well as specific health information connected to the participants’ journey through pregnancy and first year port partum. The intervention group will have access to a variety of resources associated with gestational weight gain and postpartum weight management, infant feeding and active play, and diabetes prevention. The intervention group is also able to connect with other women going through pregnancy and postpartum care using the virtual social network feature in the smartphone application.
More specifically, the intervention will consist of the following six components (see table 1 below for an illustration of the different steps included in the intervention): (1) two video synchronous sessions, the first at enrolment and the second between 4 and 8 weeks postnatal. These sessions typically last 45–50 min where health goals are discussed. This is mediated through the live-video feature in the Liva mHealth coaching app. If the woman is diagnosed with GDM, there will be an opportunity for a third 15 min synchronous coaching session to review and adjust any lifestyle goals to align with the individual’s diabetes management plan; (2) asynchronous mHealth coaching that uses a combination of text and video messaging exchanges between the mHealth coach and participant. The pregnancy interactions will be once a week for the first 4 weeks. The mHealth coaching then becomes biweekly for 2 months and then monthly until birth. The postpartum interactions will be after the synchronous coaching session. These will happen biweekly for 1 month, to support the woman to ease back into mHealth coaching contact after the birth. After that it will be weekly for a month, then biweekly for a month prior to monthly check-ins with the mHealth coach. However, this will be arranged with the woman during the postpartum synchronous coaching session and will be adapted accordingly. From 6 to 12 months post partum, asynchronous coaching will happen monthly; (3) Automated push notifications are sent out to participants. These will include messages prompting the individual to follow through on the goals they have set for themselves, reminders to register goal achievements and motivational messages when they have accomplished their goals. While these are standardised messages, they will be tailored to the individual’s goals, child feeding practice and preferences. (4) Participants will receive personalised educational content from their mHealth coach during the asynchronous coaching sessions. This content will cover topics within breast feeding, healthy eating, physical activity, emotional well-being and best practice formula feeding. Each mHealth coach will continuously assess what content is relevant before sending it to the women, (5) Participants will receive automated push notifications referring to additional content available in the Liva app. The content push notifications will be active once a week during the weeks that no asynchronous mHealth coaching occurs. These push notifications will connect participants with specially designed online resources providing recipes, information, tips for food and activity choices, breastfeeding resources and links to relevant support agencies; (6) Participants will also have access to a virtual social network, through the mHealth coaching app, with other women participating in the study. This will enable social engagement and support, as well as the capacity to connect physically for shared activities, self-organised in each study site.
Overview of intervention time points
The mHealth coaching manual will detail the a priori behaviour change techniques to be used by the coaches in the synchronous and asynchronous coaching interactions at different determined time points. The specific techniques to be used in different problem-solving scenarios (eg, overcoming barriers, relapse management) will be outlined. All coaching interactions, synchronous and asynchronous, will be recorded, in either video or text format as per the type of interaction. A sample of these interactions will be coded and compared with the coaching manual, on an ongoing basis, to provide a continuing feedback fidelity process to the mHealth coaches. For a random sample of participants, all coaching interactions will be coded against the specified coaching manual to generate a fidelity index, so that treatment fidelity can be examined in relation to participant outcomes. The app is based on the integration of three previous successful RCTs covering different aspects of antenatal and postnatal care. These studies showed the acceptability, relevance and efficacy of each of these components. These interventions were then joined together and combined with the Liva app and coaching approach, which has demonstrated efficacy through a number of trials. This new combined intervention programme was then trailed in a pilot programme with women in the identified at risk groups in Dublin. Their experiences and feedback on the programme were used to finalise the approach and content of the programme.
The telehealth coaching is a goal-directed activity, in so far as it is focused on the attainment of specific outcomes that are valued and defined by the individual being coached. Regardless of the goal, it is a partnership-based, relationship-driven, collaborative approach, focused on the implementation of a plan to strategise, discuss potential solutions, motivate, overcome barriers and move the ‘coachee’ towards their desired outcome. This approach is encapsulated in Whitmore’s Goal-Reality-Options-Wrap-Up model, an autonomy-promoting tool used by Liva coaches to guide the patient in developing their own conclusions through the stages of change described in the transtheoretical model. Moreover, user goals are rooted in integrative health and well-being outcomes.26 The Liva programme’s integrative model of theories and BCS aligns with NICE guidelines for general and individual approaches to behaviour change interventions.27 Furthermore, this alignment has been independently assessed for the design of the Liva programme as part of the review of the UK’s National Health Service (NHS) Diabetes Prevention Programme, with good fidelity results.28
Usual care
The control group receives usual care according to the maternity care at each site.29 In addition, the control group participants will be provided with links to standard information sources on gestational diabetes and lifestyle. Both intervention and usual care will receive electronic newsletters updating them on the study’s progress.
Data
Trial design
B2B&Me is a multicentre single-blind randomised controlled implementation trial with the aim of testing the innovation of a mHealth app and personalised health coaching with integrated health service screening for high-risk women in pregnancy, until 1-year post partum. The details of the trial are reported elsewhere30 but are briefly accounted for here. The study will aim to recruit around 800 women across four hospital sites; National Maternity Hospital Dublin, Ireland; San Cecilio University Hospital Granada, Spain; Southmead Hospital Bristol, England; Monash Medical Centre, Melbourne, Australia. The intervention will be implemented at each hospital site with pregnant women aged 18 and older attending maternity services screened for risk of developing GDM using the validated Monash GDM Screening Tool31 around their first antenatal visit. Individuals scoring 3 or higher on the tool will be invited to participate if they meet the inclusion and exclusion criteria below.
The inclusion criteria are women attending a participating maternity service for maternity care; scoring of 3 or higher on the Monash GDM screening tool; smartphone owner and not currently in a health behaviour change clinical trial. The exclusion criteria are previously diagnosed diabetes (type 1 or type 2); greater than 24 weeks gestation; current multiple pregnancies (eg, twin, triplets); cancer (not in remission); severe mental illness, substance abuse or myocardial infarction in the last 3 months; difficulty with using the English language for the Irish, English and Australian sites and Spanish for the Spanish site; and not owning a smartphone capable of hosting the intervention app.
At the baseline visit, demographics will be collected via questionnaire including maternal age, ethnicity, gravidity, parity, relationship status, educational attainment, employment status (of the participant and relevant partner), housing status, childcare responsibilities and prior medical history. Maternal height (cm) and weight (kg) will be extracted from medical records and participants will be invited to weigh themselves weekly on a Bluetooth-enabled scales provided by the study. Blood pressure will be measured during the baseline visit. Online questionnaires will be completed within 1 week of the baseline visit. The questionnaires will collect data on diet, physical activity, breastfeeding attitudes, health status (EQ-5D-5L), psychological health, sleep quality, health literacy and willpower. The questionnaires will be completed within a week of final study visit (12 months post partum). Additional questionnaires on infant development, health status, diet and physical activity as well as anthropometry on infant length (cm), weight (kg) and head circumference (cm) will be completed at the final visit. At 3, 6, 9 months, a questionnaire will be completed on healthcare visits and out-of-pocket costs post partum.
Effect outcomes
The primary outcome of the trial is a reduction of 0.8 kg/m2 maternal BMI in the intervention group 12 months post partum.30 The two main health outcomes for the economic evaluation will be health-related quality of life for the mother measured using the quality-adjusted life-years (QALYs) approach and maternal body weight (kg) 12 months post partum. QALYs are a summary health outcome measure routinely used for economic evaluation integrating quantity and quality of life into a single index.32 QALYs will be calculated based on responses from the 5-level EQ-5D version (EQ-5D-5L) questionnaire sent out to patients in the clinical trial and country-specific value sets will be applied.33–36
Effects (QALYs and weight) for the economic evaluation will be measured on individual patient levels at baseline and 12 months post partum. Additional effect outcomes (maternal and infant) can be employed if necessary, depending on the analysis of the trial clinical effects.
Cost outcomes
The CE analyses will be performed from a health sector perspective. Cost categories for the economic evaluation include the value of all antenatal healthcare consumption, postpartum healthcare consumption related to diet, activity and weight for both baby and mother, costs of pharmaceuticals in pregnancy and the cost of the intervention itself.
Information on utilisation of health services and utilisation of pharmaceuticals will be collected at the individual level. The antenatal services will be captured from the electronic case report forms maintained by hospital sites. If participants visited hospitals or health providers other than the trial sites, the number of diagnostic and treatment procedures will be obtained based on self-report. Information on the type of visit, frequency and out-of-pocket postpartum healthcare visit costs relating to diet, physical activity and weight management for the participant and their infant will be collected through online questionnaires at 3 months, 6 months, 9 months and 12 months post partum. Valuation of the resources identified and measured will be done using country-specific unit costs for healthcare services. Each contact (face-to-face visit, phone contact, etc) will be assigned a unit cost based on the type of visit and type of healthcare professional. Costs for the intervention itself will be collected by the company providing the smartphone application (Liva Healthcare) and will contain costs related to the implementation of the application in participating countries healthcare systems. These per-patient costs consist of time providing the intervention, costs running the technology, as well as costs for training and supervising health coaches. Costs will be presented in 2024 Euros.
Indirect costs (productivity losses) will not be collected during the trial. Therefore, the (short-term) CE analysis will only include costs for healthcare consumption (healthcare visits and pharmaceuticals) during pregnancy and 1-year post partum as well as costs for the intervention. It is assumed that the productivity losses incurred during the trial period are negligible due to maternity leave after pregnancy. However, these costs are planned to be collected from the published literature and included in the long-term CE analysis to inform the long-term CE of health-coaching support for women with high-risk pregnancy.
Methods and analysis
CE analysis
Well-established health economic methods will be employed in order to estimate the CE of the clinical trial.37 A CE analysis enables the calculation of gains in treatment effect of a specific intervention (the B2B&Me intervention in this case) with its additional costs and compares the treatment effects and costs to a comparator (usual care in this case). Comparing treatment effects and costs between two alternatives results in an ICER will be calculated as follows:
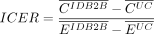
where  and
and  are the mean costs among participants in the B2B&Me and usual care arms while
are the mean costs among participants in the B2B&Me and usual care arms while  and
and  are the mean effects (QALYs or body weight). Depending on the effect measure chosen, the ICER expresses the additional costs per unit decrease in body weight or per additional QALY gained if B2B&Me is used by pregnant women at risk of gestational diabetes. Concluding whether or not the intervention or the comparator is the cost-effective alternative depends on the societal willingness to pay (for a specific country), that is, if the ICER is below the CE threshold the intervention will be regarded as cost-effective.
are the mean effects (QALYs or body weight). Depending on the effect measure chosen, the ICER expresses the additional costs per unit decrease in body weight or per additional QALY gained if B2B&Me is used by pregnant women at risk of gestational diabetes. Concluding whether or not the intervention or the comparator is the cost-effective alternative depends on the societal willingness to pay (for a specific country), that is, if the ICER is below the CE threshold the intervention will be regarded as cost-effective.
Short-term CE analysis
Short-term CE analysis will be employed using data from the clinical trial. The short-term CE analysis is planned to start in July 2024 and end during 2024. The base case CE analysis will be conducted for Ireland. Conducting analysis employing data from one trial country will result in a clear picture of the CE of the intervention and support decision-makers in decisions surrounding implementation. The results of the analysis will conclude if the B2B&Me intervention is a cost-effective alternative to usual care for pregnant women at risk of gestational diabetes in Ireland.
Long-term CE analysis
A clinical trial and the subsequent data collection often spans over a relatively short period of time.38 Health economic modelling can be employed so that results from clinical trials are extrapolated beyond the reach of clinical data and thereby providing important insights into potential long-term benefits (and costs) of an intervention. To estimate the long-term CE of the B2B&Me intervention, a Markov cohort model will be employed.38 The long-term CE analysis is planned to start January 2025 and end June 2025.
Sensitivity analysis
We plan to perform both deterministic sensitivity analysis as well as probabilistic sensitivity analysis for both short-term and long-term CE analyses.
Deterministic sensitivity analysis
Deterministic sensitivity analysis will be performed both for the short-term and the long-term CE analysis.39 The deterministic sensitivity analysis for the 1-year CE analysis will be performed by recalculating and presenting the ICER for different subgroups. Further, separate CE analyses will be conducted for each trial site. Country-specific estimates will contribute to a comparative analysis of the health systems and contextual factors affecting CE across countries. One-way deterministic sensitivity analysis will be performed for the long-term CE analysis by varying parameters in the simulation model.
Probabilistic sensitivity analysis
In order to investigate the probability of achieving an ICER that falls below a specific CE threshold, probabilistic sensitivity analysis can be performed.40 41 The results from the probabilistic sensitivity analysis will be presented in a CE acceptability curve (CEAC) and a CE plane.42 The CEAC displays the estimated probability that the B2B&Me intervention will be deemed cost-effective compared with usual care at any given CE threshold level for the ICER.43 44 The CE-plane presents all incremental effect and incremental cost pairs that are produced by the bootstrapping procedure graphically and illustrates whether most of the pairs result in CE for the intervention or the comparator.
Missing data
Participants will be followed up at specific points in time, but some participants may be lost to follow-up. In addition, data may be missing for some participants for example if the EQ-5D-5L questionnaire is not filled out at a follow-up point. These problems will be handled by applying multiple imputation methods.45–47
Discounting
In analyses where the time horizon is relatively long, costs accruing in the future are typically discounted to their present values. Likewise, future utility is worth less today. In both cases, present values can be calculated using the rate of interest and the rate of time preferences, respectively.38 In the health economic literature, costs and effects are usually discounted by annual rates ranging between 0% and 6%, for both costs and health effects.48 Costs and effects in the long-term CE analysis will be discounted using a discount rate of 4%.49
Patient and public Involvement
This protocol paper describes a health economic evaluation, which uses patient data but does not involve patients directly. We refer to the published protocol paper for more information on patient and public involvement.30
Ethics and dissemination
The National Maternity Hospital Human Research and Ethics Committee was the primary approval site (EC18.2020) with approvals from University College Dublin HREC-Sciences (LS-E-20-150-OReilly), Junta de Andalucia CEIM/CEI Provincial de Granada (2087-M1-22), Monash Health HREC (RES-20-0000-892A) and NHS Health Research Authority and Health and Care Research Wales (HCRW) (21/WA/0022). Informed consent will be obtained from all subjects and/or their legal guardian(s). The results from the health economic analysis will be disseminated in a scientific and peer-reviewed manuscript submitted to a relevant journal, through conference presentations and through different channels for communication within the Bump2Baby project.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to acknowledge the contribution of the members of the IMPACT DIABETES B2B Consortium: Associate Professor Mary Codd, Associate Professor Ricardo Segurado (University College Dublin), Professor Helena Teede, Associate Professor Cheryce Harrison, Associate Professor Jacqueline Boyle, Dr Georgia Soldartos (Monash University), Professor Cristina Campoy, Dr Mercedes Bermúdez, Assoc. Professor Alberto Puertas, Dr Francisca S. Molina, Dr María García-Ricobaraza (University of Granada), Dr Nanna Husted Jensen (Aarhus University), Dr Ditte Laursen, Elena Rey Velasco (Liva Healthcare Healthcare), Professor Timothy Skinner (University of Copenhagen).
References
Footnotes
X @oreillysharleen
Collaborators We would like to acknowledge the contribution of the members of the IMPACT DIABETES B2B Consortium: Assoc. Prof. Mary Codd, Assoc. Prof. Ricardo Segurado (University College Dublin), Prof. Helena Teede, Assoc. Prof. Cheryce Harrison, Assoc. Prof. Jacqueline Boyle, Dr. Georgia Soldartos (Monash University), Prof. Cristina Campoy, Dr. Mercedes Bermúdez, Assoc. Prof. Alberto Puertas, Dr. Francisca S. Molina, Dr. María García-Ricobaraza (University of Granada), Dr. Nanna Husted Jensen (Aarhus University), Dr Ditte Laursen, Elena Rey Velasco (Liva Healthcare Healthcare), Prof. Timothy Skinner (University of Copenhagen).
Contributors LPN planned and wrote the manuscript with the support of EC and KV. FM, HTM, SO'R, AD, CB, TCS, EC and KV have been involved in critically revising the manuscript, rewritten parts of the manuscript, have given final approval of the version to be published and agree to be accountable for all aspects of the work. EC, AD and TCS have contributed substantially to the revised version of the manuscript. All authors read and approved the final manuscript.
Funding This project is funded by the European Union Commission Horizon 2020 grant entitled 'Implementation Action to Prevent Diabetes from Bump 2 Baby (IMPACT DIABETES B2B)' under grant agreement 847984, with collaborative National Health and Medical Research Council, Australia cofunding under grant number APP1194234. The project is sponsored by University College Dublin.
Disclaimer The funders and the sponsor have no role in the design of the study, the collection, analysis and interpretation of data, or in the writing of the manuscript or decision to publish.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
↵In this paper we refer to ‘women’, ‘participants’ or ‘patients’ according to the trial inclusion criteria stating ‘women attending a participating maternity service for maternity care’.

