Article Text
Abstract
Introduction Clinical assessment in emergency departments (EDs) for possible acute myocardial infarction (AMI) requires at least one cardiac troponin (cTn) blood test. The turn-around time from blood draw to posting results in the clinical portal for central laboratory analysers is ~1–2 hours. New generation, high-sensitivity, point-of-care cardiac troponin I (POC-cTnI) assays use whole blood on a bedside (or near bedside) analyser that provides a rapid (8 min) result. This may expedite clinical decision-making and reduce length of stay. Our purpose is to determine if utilisation of a POC-cTnI testing reduces ED length of stay. We also aim to establish an optimised implementation process for the amended clinical pathway.
Methods and analysis This quality improvement initiative has a pragmatic multihospital stepped-wedge cross-sectional cluster randomised design. Consecutive patients presenting to the ED with symptoms suggestive of possible AMI and having a cTn test will be included. Clusters (comprising one or two hospitals each) will change from their usual-care pathway to an amended pathway using POC-cTnI—the ‘intervention’. The dates of change will be randomised. Changes occur at 1 month intervals, with a minimum 2 month ‘run-in’ period. The intervention pathway will use a POC-cTnI measurement as an alternate to the laboratory-based cTn measurement. Clinical decision-making steps and logic will otherwise remain unchanged. The POC-cTnI is the Siemens (Erlangen Germany) Atellica VTLi high-sensitivity cTnI assay. The primary outcome is ED length of stay. The safety outcome is cardiac death or AMI within 30 days for patients discharged directly from the ED.
Ethics and dissemination Ethics approval has been granted by the New Zealand Southern Health and Disability Ethics Committee, reference 21/STH/9. Results will be published in a peer-reviewed journal. Lay and academic presentations will be made. Māori-specific results will be disseminated to Māori stakeholders.
Trial registration number ACTRN12619001189112.
- ACCIDENT & EMERGENCY MEDICINE
- Myocardial infarction
- Adult cardiology
- Ischaemic heart disease
- Emergency Departments
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
- ACCIDENT & EMERGENCY MEDICINE
- Myocardial infarction
- Adult cardiology
- Ischaemic heart disease
- Emergency Departments
Strengths and limitations of this study
The study size and breadth across emergency departments of different sizes and demographics improves generalisability and allows exploration of multiple secondary outcomes.
A pragmatic study allows for increased generalisability as it reflects the real-world challenges of implementing change.
The challenges of implementing change may impact the analysis of the primary outcome measure of length of stay through things such as delays caused by IT development, laboratory approvals, nurse training, site-specific requirements or unforeseen local or national events (eg, such as a pandemic).
We are unable to limit this to patients investigated for possible acute coronary syndrome only, and so we will include patients also investigated for myocarditis or pulmonary embolism for example.
The size prevents adjudication of outcomes, so hospital coding for myocardial infarction, previously shown to have 98% agreement with adjudicated outcomes for major adverse cardiac event, must be relied on.
Introduction
Background and rationale
Current clinical assessment pathways for possible acute myocardial infarction (AMI) involves pathways incorporating clinical history, ECG and one or more blood tests to measure cardiac troponin (cTn) concentration. During 2015–2016 all emergency departments (EDs) in New Zealand implemented structured assessment for patients with suspected AMI with accelerated diagnostic pathways (ADPs) in place (Improving Care processes for patients with suspected Acute Coronary Syndrome (ICare-ACS)).1 There are minor regional differences between pathways. For example, because of higher incidence of AMI in some populations, some use telemetry monitoring for all Māori (indigenous people of New Zealand), Pacific peoples and Indo-Asian, irrespective of risk factors. All EDs in this quality improvement initiative now also use an early ‘single-sample’ low-risk stratification procedure as a component of that pathway. Using this approach, a patient may be considered as low-risk and suitable for early discharge based on evaluation of their ECG, history, signs and symptoms and a very low high-sensitivity (hs) cTn concentration. This practice has been established on the basis of multinational meta-analyses of safety and efficacy.2 3
Despite the time-efficiencies that this strategy creates, rapid decision-making is limited by the overall turn-around time (TAT). In this project, this will be defined as the time taken between blood-draw to electronic availability of the assay result for viewing by the physician (the vein-to-brain time). The preanalytic times required to transport a blood sample to a central laboratory, register it and prepare it for measurement are significant components of the overall TAT. Lack of immediate availability of results delays decision-making and action by a clinician. Point-of-care cardiac troponin I (POC-cTnI) assays use whole blood on an analyser that can be placed near to the patient and provide a result within ~8 min. POC-cTnI use has been proposed to expedite decision-making and may reduce ED length of stay (LOS).4–6
Contemporary POC-cTnI assays use technology that lacks the high analytical precision shown by hs-cTn assays run on analysers based in central hospital laboratories. This made them unsuitable for accurate low-risk stratification of AMI after a single blood sample. Several manufacturers have now received regulatory approval for POC-cTnI assays that have similar analytical precision at low-concentrations of cTn as central laboratory hs-cTn assays and use an unspun whole blood sample.6–9 Consequently, low-risk stratification of AMI with a POC-cTnI assay using a single low-concentration threshold is now possible.9
Objectives
The aim of this pragmatic quality improvement initiative is to assess the impact of a hs POC-cTnI assay in real-life care in the EDs of multiple hospitals. We also aim to establish an optimised process for including POC-cTnI within clinical pathways to achieve effective implementation.
Trial design
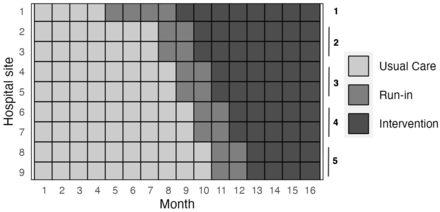
This quality improvement initiative utilises a pragmatic multicentre stepped-wedge cluster randomised design (see figure 1).
Stepped wedge cluster randomised design: a usual-care period for each site followed by a run-in phase and a monitored intervention phase. Shown is the design for the maximum number of hospitals able to participate.
This manuscript has been reported in accordance with the Standard Protocol Items Recommendations for Interventional Trials (SPIRIT).10
Methods: participants, interventions and outcomes
Initiative setting
Up to nine acute care hospitals across New Zealand will be included. All the hospitals involved have some degree of association with a medical school. These will range from small to large in size, and with varied population demographics, all of which use central laboratory hs-cTn assays (table 1: sites scoped). The final choice of hospitals will depend on preparedness, availability, willingness to participate and resources. Patients presenting from the community to the ED with chest pain do so mainly by self-presentation but there are also presentations via General Practitioners. Patients may arrive by ambulance or their own transport. Initial medical assessment is usually performed by doctors from the Emergency, Cardiology and Internal (General) Medicine services. Clinical experience of the physicians attending ED cases ranges from house officer (resident) to consultant (attending) specialists.
Sites scoped as eligible for participation
All participating hospitals have a local clinical pathway for the assessment of patients with possible AMI, based on national and regional practice guidelines (see Usual-care below).11 These pathways were evaluated by the ICare-ACS project and then introduced into Ministry of Health (MoH) National Service Frameworks. The extended project team then facilitated their implementation across EDs in New Zealand during 2015–2016.1 Since then, pathways have changed to include a single sample low-risk stratification approach when cTn is less than a prespecified threshold taken a minimum duration after first/worst pain, and there are no other high-risk features (such as ischaemic ECG changes or unstable clinical finding, eg, abnormal vital sights or angina of increasing frequency or severity; see exemplar pathway in online supplemental figure 1). These pathways may include minor adaptations to suit local settings, resources and demographics (eg, high Māori population or no on-site Cardiology service).
Supplemental material
Clinical pathways are the translation of practice guidelines and provide a plan of care suited to the local health system. They incorporate factors such as resource availability and consensus among local experts. Clinical pathways are structured, multidisciplinary inventories of actions that meet any three of the following criteria:12
Used to support guidelines or evidence into local practices.
Detail the steps in a course of treatment or care.
Have a time frame or criteria-based progression (ie, steps are taken if or when designated criteria are met).
Aim to standardise care for a specific clinical problem or outcome.
To these we add the following:
Reduce barriers to care and ensure equity of treatment for current marginalised communities.
Clinical pathways have been shown to reduce complications, decrease LOS and decrease hospital costs.12 13
Participants (eligibility criteria)
All patients presenting to the EDs of participating hospitals in whom attending clinical staff order cTn test(s) because there is perceived need to investigate for possible AMI alone or part of a differential diagnosis.
Inclusion criteria
Adults (≥18 years of age).
cTn test performed.
Included will be consecutive adult patients who present acutely to the local hospital ED with recent onset symptoms due to possible AMI in whom the attending clinician intends to measure cTn. This will remain unchanged between phases of the project. In keeping with the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain such symptoms may include (but are not limited to): ‘pain, pressure, tightness, or discomfort in the chest, shoulders, arms, neck, back, upper abdomen, or jaw, as well as shortness of breath and fatigue’.14 The decision to order cTn may be made by medical and/or nursing staff. The primary analysis will be based on all presentations because we aim to assess real-world impact of POC-cTnI on the local health system as a whole. A sensitivity analysis will be per patient (first presentations only).
Exclusion criteria
Died within the ED.
Transfer from other hospitals.
Design (and intervention)
This quality improvement initiative utilises a pragmatic multi-centre stepped-wedge cluster randomised design (figure 1).
Three phases will occur at every hospital as follows: (1) a usual-care phase, (2) a run-in phase and (3) an intervention phase; these phases are described in detail below.
There will be 1 month intervals between sites changing phases. The duration of usual-care and intervention periods will be at least 4 months at each site. Because of the use of a sentinel site with a longer run-in phase and earlier intervention phase, and the same starting date for all sites, the usual-care phase will be longer in other sites than their intervention phase.
Usual-care (control) phase
The hospitals (within clusters) continue to apply their usual pathway that uses central laboratory hs-cTn thresholds to guide decision-making.
Run-in phase
This is defined as a 2 month period (4-months for the sentinel site) which is excluded from primary analysis. The ‘run-in’ period allows for gradual adoption and embedding of the ‘intervention’ clinical pathway, including familiarity, confidence and competence in using the adapted clinical pathway. Implementation processes will be developed with local stakeholders to reflect local practice and incorporate lessons learnt from other hospitals.
Intervention-evaluation phase
In the intervention phase, the only modification to the decision-making logic of existing usual-care clinical pathways is the use of a POC-cTnI measurement within the ED in place of the central laboratory hs-cTn measurement. The intervention will remain in place in all sites for a minimum 4 months following the end of the run-in period at the final site(s). POC-TnI will be the preferential troponin test used in the ED instead of central laboratory cTn testing. The laboratory test may still occasionally be used when:
The patient is not suitable for early discharge and requires further investigation for potential AMI (see online supplemental figure 1), prompting patient admission and further monitoring of cTn using the central laboratory assay.
The patient has recently had a cTn measured using the central laboratory test, so that further measured concentrations can be compared with that baseline.
There is an error message from the POC analyser.
POC analyser(s) become unavailable because of malfunction.
In these cases, central laboratory cTn measurement will be made for initial and any subsequent measurements (as per the local pathway used in the Usual-care period). The final decision regarding which assay to use, and clinical decision-making, for an individual patient is at the treating clinicians discretion, incorporating all other clinical information and advice given by colleagues.
The anticipated effect of the intervention on patient flow is shown in figure 2.
Anticipated patient flow through each stage of the initiative. POC, point-of-care.
High-sensitivity point-of-care troponin test
The POC-cTnI is the Siemens (Erlanger, Germany) Atellica VTLi hs-cTnI assay. All measurements will take place on whole blood from venepuncture. The VTLi assay utilises secondary cTnI antibodies directed at epitopes 23–29, 87–89 and an anti-cTnC antibody.7 The characteristics of this assay are described in table 2.
Characteristics of Siemens Atellica© VTLi assay36
Change management
The design is an established strategy for the introduction and assessment of practice changes in the field.1 15–18 The design allows for refinements in the delivery of the change of practice (but not the pathways themselves) between steps to allow refinement of the implementation process. We will use tools from the Institute for Health Improvement. First, the Plan-Do-Study-Act (PDSA) cycle, to introduce change and to adapt to subsequent findings in real-time clinical practice and, second, a Framework for Spread: From Local Improvements to System-Wide Change.19 20
Christchurch hospital will act as the sentinel cluster (site 1). Christchurch has the most experience of all sites at implementation of change of practice of chest pain ADPs.1 21–23 The intensive effort at this first site will provide early lessons to inform later sites and minimise implementation challenges. The experience at the Christchurch site will therefore act as an initial PDSA cycle (online supplemental figure 2). For this reason, Christchurch will have a longer run-in period, at least 4 months. Prior to or during the beginning of run-in phase in Christchurch, several key steps will take place: (1) nurses will be trained in the use of the POC-cTnI and standard operating procedures for nurse training around the country established, (2) measurements will be obtained on the POC device in parallel to the laboratory assay, but not initially used for clinical decision-making (see step 4 below), (3) processes will be established for linkage of results to the electronic medical record, and, finally, (4) during run-in physicians will begin to use the POC-cTnI for clinical decision-making. Other hospitals will be randomised to clusters starting the run-in period at different dates (figure 1).
Outcomes
Quality improvement objectives
Determine if POC assay testing changes ED LOS (part A).
Establish optimised implementation process for the amended clinical pathway (part B)
Part A outcomes
Primary outcome
ED LOS in minutes: the duration from presentation to ED to discharge home or admission to a hospital ward (as recorded on hospital electronic patient management system).
Secondary efficacy outcomes
ED LOS for all patient presentations discharged directly from the ED.
ED LOS for presentations discharged after a single cTn result.
ED LOS for presentations discharged after a single cTn result below a single test low-risks stratification threshold.
ED LOS for presentations who are discharged after ≥2 troponins.
Proportion of presentations discharged after 1, 2, 3, 4, 5 and 6 hour.
The number of cTn tests per month for both POC and laboratory assays.
The proportion of presentations undergoing cTn testing with a clinical diagnosis of AMI.
Secondary safety outcomes
Major adverse cardiac event (MACE) rate per presentation within 30 days for patients discharged directly from ED and the rates for each individual component of MACE.
MACE is defined as follows:
cardiac death.
AMI determined by ICD10-Australian Modification codes:24
STEMI: I21.0 or I21.1 or I21.2 or I21.3
NSTEMI: I21.4 or I21.9 or I22.0 or I22.1 or I22.8 or I22.9.
MACE rate within 30 days for all presentations.
MACE rates within 1 year of presentation for those discharged directly from ED and for all patients.
Note: Because cause of death is not coded, the cause of death will be adjudicated by two independent consultant specialists for any patients discharged from ED who died within 1 year.
Planned further analyses are outlined in the online supplemental file.
Part B outcomes
A mixed-methods case study research design which includes both qualitative and quantitative methods of data collection will examine the implementation process and map the findings to the Consolidated Framework for Implementation Research.25 We will examine the barriers and facilitators to the implementation and use of POC cTn testing by end-users in the ED. This will allow the creation of specific recommendations to enhance implementation of POC cTn testing in the ED. This will have wider implications for POC testing in the ED in general. These findings will be reported separately.
Participant timeline
Each participant will be followed for 1 year after presentation. Each hospital will participate for ~17 months depending on the final number of clusters (figure 1). Any patient with MACE identified by coding within 30 days of having been discharged from the ED will be formally audited by the local hospital clinical team and adjudicated by two independent physicians. The start date for the first presentation is 1 February 2023 and anticipated final hospital presentation 31 December 2024.
Sample size
A reduced LOS of ≥15 min in the low-risk patients (without AMI) would be clinically meaningful because of the large numbers of patients impacted. Christchurch hospital has been running a single-sample low-risk stratification pathway since 2018; 2019 data gave a mean ED LOS for all patients with a first ED cTn within 90 min of 4.1 hour and SD of 1.7 hour. Using the equations provided by Hemming et al, we calculated that at an α = 0.05 with a power of 90% and assuming five clusters and an intracluster correlation coefficient ρ=0.1 (moderate; ICare-ACS had ρ ∼ 0.01), then a sample size of 5785 patients is required.26 27 If all sites complete the initiative, we anticipate up to >70 000 participants (depending on site randomisation sequence and total usual-care period) (table 1).
Power is also sufficient to also assess the key secondary outcome of ED LOS of patients discharged after a single cTn result below a single sample low-risk stratification threshold assuming that at least 15% of the patients fall into this category. We anticipate that ~35% will fall into this category.
Māori have a higher incidence of AMI and present at a younger age than non-Māori.28 With an estimated 9000 Māori in the project, there is adequate power for the key subgroup analysis of ethnicity.
Safety: the rate of 30 days MACE in the ICare-ACS study was 0.44%1; 1% is considered acceptable; 8200 patients are adequate to detect a difference of 0.56% (1–0.44) between arms with an α = 0.05 and a power of 90%.
Methods: assignment of interventions
Site randomisation (sequence generation)
Christchurch is the sentinel site and will be the first to introduce the intervention. Randomisation (computer-generated) of the other clusters to begin the intervention 1 month apart will be conducted by the statistician, JWP, using the base R function sample.29
Methods: data collection, management and analysis
Data collection methods
Patients will be identified at each hospital site as those having cTn measurements within the ED. The National Health Index (NHI) identifier is a unique identifier which links the admissions, hospital blood measurements and all mortality events of every person presenting to a New Zealand hospital. The NHI will be used to identify linked event and outcome data including 30 days mortality and readmissions. These will be obtained from the MoH National Mortality data set, National Minimum data set of hospital events and National non-admitted patient collection. We successfully used this approach in the ICare-ACS study.1 Any occurrences of MACE will be identified using ICD-10 codes of readmissions over 30 days following the presentation and mortality records.
All data are routinely collected prospectively and we believe that there will be no bias or decline in data quality as a consequence of accessing this data retrospectively.
Additional monitoring: we will closely observe the change management at all hospitals. This will include documenting any specific changes to the clinical pathways, including identifying if changes are made through incorporating tikanga Māori into the process. The collation of this information will then be made available to other hospitals, not part of this project, to inform any changes to their clinical pathways.
Data management
The statistician, JWP, will be responsible for data management. Data will be transferred by sites, and kept on, secure servers operated by Christchurch Hospital. All analysis will be conducted with R.29
Statistical methods
Since this is a quality improvement initiative implementing a new process, we will report using the Standards for Reporting Implementation studies (StaRI).30 Additionally, we will use the CONSolI-Dated critERtia for strengthening the reporting of health research involving Indigenous Peoples (CONSIDER) guidelines.31 Patient demographic summary data will be limited to age, sex and ethnicity which will be reported as mean±SD and n(%). We will summarise time from ED arrival to first blood draw, time between blood draws, numbers and proportions with only one ED blood draw and rates of MACE events.
The primary analysis for LOS will use a generalised linear mixed model to predict ED LOS comprising quality improvement arm (before implementation of POC and after), hospital site, cluster, time of presentation (in days) since initiative start, season and shift. Cluster, hospital site are random effects (to allow for variation in ED LOS between sites and clusters), the rest are fixed effects. The statistical analysis plan will be finalised before the data are collected and collated. Prior to final analysis, the ED LOS will be assessed for skewness and, if necessary, transformed for before use in the model. The beta-coefficient for quality improvement arm will be presented with 95% CI.
The primary analysis will be based on the beginning of the randomised intervention period or the actual date of the start of clinical decision-making with the POC-cTnI if later than the randomised date for each site.
There will be single-site interim analyses that utilise presentations prior to the intervention phase and compare the same period before and after the beginning of use of the POC for decision-making (started during run-in). The purpose is twofold, (1) as a pragmatic QI with PDSA cycles, this may highlight areas for further education of staff and (2) each site will need to make decisions about the continued use of the POC or not prior to the end of the QI. The first interim analysis from Christchurch hospital will be published.
Methods: monitoring
Data monitoring
From a data monitoring perspective, there is no requirement for a Data Monitoring Committee. The data regularly provided by hospitals to the MoH on ED presentations undergo cleaning. From a safety perspective, this is part of routine care and is described below.
Harms
For the purpose of the evaluation of this quality improvement initiative in patients discharged from ED by the pathway, any death within 30 days for any reason related to ischaemic heart disease and/or the patient’s original presentation will be considered a serious adverse event.
Serious adverse events
These will be reported to the principal investigator within 7 days of the event. Reports will include the following:
Grade of event
Date and time of ED visit
Details of ED investigations and biochemistry
Date and time of event
Description of event
The site champion’s assessment of the relatedness (possible, probable, or definite) to the ED visit.
A multidisciplinary clinical governance committee will review any adverse events at each site. Following review events will be acted on depending on severity and in keeping with the standard governance processes for that health institution. Any site may decide to withdraw from the study as a result of such a review, if they have safety concerns.
Auditing
An interim data extract for each individual hospital will occur prior to the end of the initial evaluation period with data from the control and run-in periods only and a simple hospital level analysis will be undertaken by the statistician (JWP) to provide information to hospital management (independent of the investigators) who need to make decisions about ongoing use of POC-cTnI after the end of the initiative evaluation period. This will include an analysis of the uptake of the POC assay and continued use of the laboratory assay.
Assessment for pragmatism
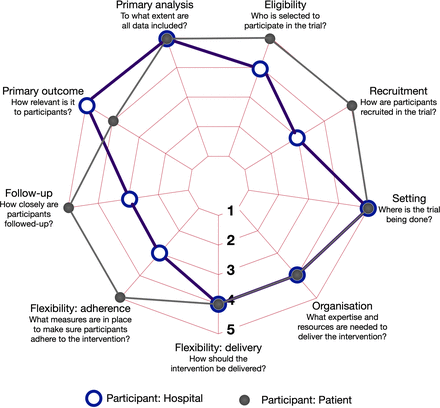
Pragmatic studies, as opposed to explanatory studies, are designed to work within real-world clinical situations. They allow for issues encountered in clinical practice. Results, therefore, take into account the complexities of routine practice.30 The PRagmatic Explanatory Continuum Indicator Summary (PRECIS-2) tool has been designed to help design pragmatic studies.32 On a 5 point scale, with 5 being the most pragmatic and 1 the most explanatory it, it asks nine domain questions. We assessed our design from both the patient and hospital site perspective on each domain using the guidance provided by PRECIS-2 (figure 3).
PRECIS-2 diagram demonstrating how pragmatic the initiative is from the hospital (open circles) and patient (closed circles) perspective; 5 is very pragmatic, 1 is very explanatory. PRECIS-2, PRagmatic Explanatory Continuum Indicator Summary.
Methodology limitations
Without a detailed inspection of the notes of every ED presentation, we are unable to ascertain which presentations were adjudicated for possible ACS and which had a troponin measured for other reasons (eg, myocarditis or pulmonary embolism). While the run-in phase allows for some delay in starting to use the POC device at a hospital, there could still be delays due to IT development, laboratory approvals, nurse training, site-specific requirements or unforeseen local or national events (eg, such as a pandemic). The size of the study precludes blinded adjudications of myocardial infarction outcomes. However, an audit of ICare-ACS found ICD-10 codes for MACE corresponded to adjudicated MACE in 98% of cases.1 Mortality outcomes must be adjudicated to find those that are cardiac caused, but as these will be small in number this is possible.
A particular challenge with implementing change and assessing with a stepped-wedge design is that the outcome may be influenced by time varying effects.33 The presence of shift, season and time from start of study in the statistical model attempt to account for this. How this time is input into the model, as a categorical, continuous, fixed or random effect may affect the outcome. Our design and analysis approach is similar to that of Anand et al where hospitals with patients with suspected ACS and underwent a change in assessment pathway.17 A post-hoc investigation that included comparing time from study start as a cubic spline, categorical variable and as a random variable, found very little influence compared with using time as a simple linear fixed effect variable.34 Nevertheless, having time as fixed effect assumes a similar linear trend in all hospitals, which may have been the case in the Anand study, but may not be the case in this study. Therefore, we will not finalise the model until after comparing several options, using a simulated intervention and LOS data from a period preceding the present study.
This study design is known for issues where clusters split, drop-out or do not start at the expected time, or have an effect which changes over time.35 The run-in period which allows for gradual adoption (including short delays) and familiarity of the intervention pathway will help mitigate the risk of the latter two issues. We screened all New Zealand hospitals and determined that we could include up to nine hospitals with five clusters of up to two hospitals per cluster. At the time of writing six clusters of one hospital, each have agreed to participate and have been randomised. This provides a sufficient sample size for the preplanned primary and secondary analyses.
Ethics, dissemination, patient and public involvement
Ethics approval has been granted by the New Zealand Southern Health and Disability Ethics Committee, reference 21/STH/9. Māori consultation was undertaken in collaboration with the Māori/Indigenous Health Institute (MIHI), University of Otago Christchurch to seek feedback from local indigenous groups. Any protocol amendments will be reported to the trial registry ethics committee and investigators. As this was a quality improvement initiative, no individual consent is necessary. However, patients will be made aware of the initiative via the patient information sheet they would normally receive when leaving the ED. All data collected are already usually collected as part of routine clinical care. The same confidentiality and privacy arrangements governing the use of patient data within usual-care will apply. Data will be deidentified before collation for study analysis. Patient-level data will only be available to the investigator team. Summary data, which do not involve any individual patient information, will be made available to Siemens. New Zealand has a no-fault compensation scheme for all healthcare consumers, via Accident Compensation Corporation (ACC).
Dissemination policy
At the conclusion of the initiative summary, results will be provided directly to all stakeholders. A detailed manuscript will be prepared for publication in an academic journal. Lay and academic presentations will be made. Under consultation with MIHI, Māori-specific results will be prepared and made available for dissemination. Authorship eligibility will be as per publication guidelines, and no professional writers will be used. A public-facing website is planned to be created, which will include the participant information.
Ethics statements
Patient consent for publication
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @laurajoycenz
Contributors MT is the principal investigator. MT and JWP wrote the first draft of the NZ Health Research Council grant and protocol and are the primary authors of all subsequent versions. MT with assistance from LJ and JWP obtained ethics approval and sponsorship from Siemens Healthineers. JP wrote the statistical sections of the grant and manuscript with specific contributions from CF. MT, JWP and LJ wrote the final drafts of this manuscript. SP and CJL, equity experts, revised and added to the protocol and manuscript from an equity and Māori perspective. GD, SA, RWT, AMR and LJ are New Zealand cardiologists and ED specialists who contributed to the content of the protocol and revised the manuscript. RB, AJ, FSA, NM, RWT, PK, FP, LC, SJL, CM and AMR are members of the international expert advisory committee and have each advised on and content of the protocol, and revised the manuscript. NM also contributed to refining the stepped-wedge study design. All authors gave approval of the manuscript for publication.
Funding This work was supported by the Health Research Council of New Zealand grant number 19-234. Additional support in kind has been provided by the New Zealand Ministry of Health to enable connectivity between each point-of-care (POC) device and individual hospital IT systems. Support in kind was also provided by local hospitals and laboratories as part of the on-boarding of the VTLi test. Siemens Healthcare provided the POC devices and assays for the duration of the project and guidance for staff training and installation of the analysers. Additional funding was provided by Siemens, on request, to support education and project management. JP is supported by the Emergency Care Foundation. NM is supported by a Chair Award, Programme Grant and Research Excellence Award (CH/F/21/90010, RG/20/10/34966, RE/18/5/34216) from the British Heart Foundation.
Disclaimer Health Research Council (HRC) sponsorship was obtained after a competitive funding process. During this process, feedback was provided regarding the study and its design by a scientific assessment committee and by international peer review obtained by the HRC. This feedback was incorporated into the project design. Siemens Healthcare had no part in the writing of the protocol, and will play no part in the data analysis, interpretation or writing of manuscripts.
Competing interests Siemens Healthcare had no part in the writing of the protocol, and will play no part in the data analysis, interpretation or writing of manuscripts. JWP reports consultancy with Upstream, Luminoma, Siemens Healthineers, Quidel Corporation and Radiometer Health. NM has received honoraria from Abbott Diagnostics, Roche Diagnostics, Siemens Healthineers and LumiraDx and the University of Edinburgh have received research grants from Siemens Healthineers and Abbott Diagnostics unrelated to this work. LC has received honoraria/speakers’ fees from Abbott Diagnostics, Beckman Coulter and Siemens Healthineers, and institutional research grants from Siemens Healthineers, Beckman Coulter and Abbott Diagnostics unrelated to this work. ASJ reports work with all major diagnostic companies. FP reports research grants with Brainbox, Quidel; Consultancies with Abbott, Brainbox, Instrument Labs, Janssen, Osler, Roche, Siemens, Spinchip, Vifor; Stock/Ownership Interests with AseptiScope, Brainbox, Braincheck, Coagulo, Comprehensive Research Associates LLC, Comprehensive Research Management, Emergencies in Medicine LLC, Fast, Forrest Devices, Ischemia DX LLC, Lucia, Prevencio, RCE Technologies, ROMTech, ScPharma, Trivirum, Upstream. MT has received honoraria and research funding from Abbott, Alere, Beckman, QuidelOrtho, Radiometer, Roche and Siemens.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.