Article Text
Abstract
Introduction Commercial milk formula manufacturers often emphasise their role in supporting infant and young child nutrition and breastfeeding, but their commercial goals to increase volume and profit margin of formula sales conflict with these declarations. Healthcare professional associations have an important role in healthcare worker education, shaping clinical practice. When healthcare professional associations enter into financial relationships with formula manufacturers, conflicts of interest arise, which may undermine education and practice that promotes optimal infant and young child feeding. The World Health Assembly calls on all parties to avoid such conflicts of interest, but it is uncertain how often this recommendation is followed. This protocol documents a systematic method to identify funding from the commercial milk formula industry among international, regional and national associations of healthcare professionals.
Methods and analysis Using systematic search strategies in the Gale Directory Library and Google, we will identify international healthcare professional associations relevant to maternal and child health. Data regarding funding relationships with the commercial milk formula industry over the past 24 months will be extracted from the official websites or, in their absence, social media accounts by two independent analysts. The analysis will focus on the presence of conflict of interest or sponsorship policies and type of funding, such as sponsorship or payment for services.
Ethics and dissemination This study does not require ethical approval and will use data available in the public domain. The results will be disseminated through peer-reviewed journal articles, at conferences and among the healthcare professional associations.
- Social Media
- Cross-Sectional Studies
- NUTRITION & DIETETICS
- PAEDIATRICS
- Nurses
- PUBLIC HEALTH
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
This study covers a large number of healthcare professional associations (HCPAs) from various specialties relevant to child and maternal health to analyse their financial relationship with the commercial milk formula industry.
Our methodology builds on a previously applied approach and is supported by a comprehensive systematic search strategy with predefined keywords.
This study relies on cross-sectional, publicly accessible data shared by HCPAs, potentially underestimating the extent of financial relationships between commercial milk formula industry and HCPAs, also due to the challenges of identifying front organisations, especially in jurisdictions where such disclosures are not required or officially recommended.
Our study is limited by its focus on sponsors explicitly identified as commercial milk formula companies, possibly underestimating the broader impact of cross-ownership and interconnections within the food and pharmaceutical sectors.
The methodology might miss HCPAs without relevant online presence and some information might potentially be misinterpreted by the reliance on automated translation for non-English language websites.
Introduction
Breastfeeding is the best method of nourishing children, supporting the health of both the feeding mother and the child. Commercial milk formulas (CMFs, see Glossary in online supplemental material for comprehensive definitions of terms) are promoted by the industry as a suitable alternative for reasons that go beyond medical considerations. The CMF industry (see Glossary) employs marketing strategies that might incline families to introduce CMF into their infants’ and young children’s diet, often by using health claims with little or no scientific substantiation.1–3 Over 40 years ago, the marketing practices of the CMF industry were recognised and broadly criticised for posing threats to breastfeeding and infant health, prompting the introduction of the World Health Assembly (WHA) International Code of Marketing of Breast-milk Substitutes (‘the Code’) and subsequent resolutions.4 Despite this, the annual marketing expenses of this sector are on the rise, and a substantial amount of these funds is designated for sponsoring healthcare professional associations (HCPAs, see Glossary).1 5
Supplemental material
To understand why the CMF industry may be interested in funding HCPAs, we must acknowledge the role these associations play in healthcare systems and professional education. HCPAs are involved in developing guidelines that shape clinical practice, curriculums and training programmes for healthcare professionals and funding research projects. Moreover, HCPAs have access to extensive networks of healthcare workers and are recognised as trusted sources of information within medical communities. Already in 1989, the World Health Organization (WHO) and United Nations Children’s Fund (UNICEF) emphasised that ‘health worker’s professional associations should be actively involved in promoting appropriate teaching curricula for health workers and in developing socially responsible policies for encouraging and supporting breast-feeding in maternity and other health services’ (page 12).6
Partnerships with HCPAs could enable the CMF industry to influence research and development in the field of infant and young children nutrition. As a result, HCPAs are at risk of becoming channels for disseminating CMF-favourable content among their members, who, due to societal trust and regular interactions with caregivers, relay this content to families both before and after childbirth. This relationship with HCPAs offers avenues for infant formula manufacturers to share their message more broadly and authoritatively,1 strengthening their market position. At the same time, this might lead to conflicts of interest (COI) as well as undermining the independence and objectivity of HCPAs. This, in turn, could have long-term implications for breastfeeding practices and the welfare of families.7
COI in this context refers to a situation where financial or other material incentives might undermine, compromise or work against optimal health recommendations8 (see Glossary for an extended definition of COI). This often happens without an individual being aware of such bias.9 HCPAs and their members have a primary commitment to enhance maternal and child health, and there is a strong scientific consensus underscoring the health risks associated with using CMF in place of breastfeeding.10 Therefore, as the industry aims for growth and profit, it becomes vital to discern and navigate these financial COI transparently, ensuring the health and well-being of children and caregivers always remain the priority.11
To effectively avoid or manage COI and ensure the independence of expert judgement and knowledge generation, HCPAs should establish and adhere to stringent policies on the acceptance and utilisation of financial support from commercial entities, outlining clear thresholds, acceptable forms of funding and rules for managing COI. This is particularly relevant in the domain of infant feeding where COIs are common.1 12
Recognising the need to mitigate the impact of commercial influences on breastfeeding practice, the 69th WHA adopted the Ending the Inappropriate Promotion of Foods for Infants and Young Children resolution.13 This was further reinforced in 2017 when the WHO published the Guidance on Ending the Inappropriate Promotion of Foods for Infants and Young Children: Implementation Manual.14 This document states that companies marketing foods for infants and young children should not create COIs in healthcare facilities or throughout healthcare systems. It underscores the responsibility of healthcare workers, health systems, professional associations and non-governmental organisations to avoid such conflicts. These guidelines precisely define inappropriate practices and highlight that donations of any form, distribution of equipment or services from companies, either to healthcare systems or directly to healthcare workers or professional associations, constitute a COI and should not be allowed. Furthermore, these guidelines prohibit the financial support for scientific meetings and meetings of HCPAs by the infant and young child food industry.
Despite official declarations of adopting the Code in most jurisdictions, CMF marketing strategies are still directed towards HCPAs,1 5 11 as recently documented for paediatric associations.15 The aim of this protocol is to outline a broader approach, expanding the scope beyond paediatric associations to include all HCPAs relevant to maternal and child health. We detail a systematic method to identify the financial ties between the CMF industry and HCPAs based on publicly available information.
It is important to note that throughout this protocol, we use the terms ‘women’ and ‘breastfeeding’ for brevity, and because most people who breastfeed identify as women; we recognise, however, that not all people who breastfeed or chestfeed identify as women.11
Methods and analysis
This protocol for a cross-sectional analysis documents an exploratory descriptive method to identify funding relationships between the CMF industry and international, regional and national HCPAs. The primary method will be to retrieve publicly available information regarding CMF industry financial support from official websites of the HCPAs.
Adopting a previously developed method,15 we will identify: (1) COI policies or any other information on rules regarding receiving funding or donations available from the HCPAs and (2) evidence of any financial sponsorship or payment for services (see Glossary) received by the HCPAs from CMF industry, that is, CMF manufacturers or subsidiary organisations.
We define an HCPA as an organisation representing voluntary members from either regulated or self-regulated specific healthcare professions, relevant to infants and young children feeding, nutrition, breastfeeding and lactation. These members, including physicians, nurses, midwives, dietitians/nutritionists and lactation support specialists/consultants, often possess specific licences/certificates or diplomas from accredited studies or training in their home countries to ensure the protection of public health and patient safety.
Our selection of target professions is based on their crucial roles in promoting and supporting breastfeeding, as emphasised by various authoritative sources. The updated 2018 WHO and UNICEF guidelines Protecting, promoting and supporting breastfeeding in facilities providing maternity and newborn services: implementing the revised Baby-friendly Hospital Initiative (the ‘Baby-friendly Hospital Initiative’) highlighted that ‘The key clinical practices and global standards of the revised Ten Steps should be written into the standards of care for professional bodies. At a minimum, standards for nursing, midwifery, family medicine, obstetrics, paediatrics, neonatology, dietetics and anaesthesiology should be laid out as basics of care for all newborns.’ (page 27).16 The Academy of Breastfeeding Medicine and the American Academy of Pediatrics, emphasise the pivotal roles of paediatricians, obstetricians/gynaecologists and family physicians in supporting breastfeeding.17 18 The Global Breastfeeding Collective further highlights that midwives and nurses are ideally positioned to advocate for breastfeeding in various settings, given their diverse roles as neonatal nurses, community, and public health nurses/health visitors.19 Each listed profession, from nursing to anaesthesiology, interacts with caregivers and newborns in varying capacities and at different stages, making them crucial touchpoints for breastfeeding support. Lactation consultants play a special role in this context, providing specialised expertise in breastfeeding support.20
CMF is breast milk substitute, defined as any food intended as a partial or total replacement for breast milk, for use in the first 3 years of life.1 This definition includes exempt infant formulas or formulas for special medical purposes as well as supplements or fortifiers added to milk formulas, but excludes nutritional interventions such as vitamin or mineral supplements which are not given within a CMF21 (see Glossary for an extended definition of CMF).
The CMF industry refers to the sector involved in the production/distribution/sales/marketing of these formulas (see Glossary for an extended definition of CMF industry).
A previous analysis concerning CMF companies’ funding of professional paediatric associations was based on a list of national paediatric associations provided by the International Paediatric Association’s website.15 We will replicate and update these earlier findings, as well as include HCPAs representing paediatric specialities within allergy and immunology, dermatology, diabetology, endocrinology, gastroenterology, hepatology and nutrition. These additional fields of medicine were identified based on approved European, British and American specialty curricula. We recognise the variability in medical training and specialisation across different countries and use the term ‘paediatric specialty’ for simplicity. Fields such as paediatric allergy/immunology and dermatology may function either as independent specialties or subspecialties within paediatrics. For instance, in the USA, paediatric allergology and immunology stand as separate disciplines. Moreover, in certain cases, paediatrics may be a focal area within a broader medical specialty rather than a formalised educational pathway.
Additionally, we will include healthcare professionals listed by the Baby-friendly Hospital Initiative as well as: anaesthetists, family medicine physicians, obstetricians, gynaecologists, neonatologists, nurses, midwives, dietitians, nutritionists and lactation consultants. In the case of nurses, we will include both generalists and those with specialised roles, similar to the physician specialties listed above. Moreover, we will include nurses specialising in community and public health, as well as those in health visitor roles.
We will focus on identifying sponsors who identify themselves as CMF companies on their websites or in promotional content, regardless of their sector(s) of origin (dairy/food manufacturing, pharmaceuticals, consumer goods) or those featured on the List of major companies and their main brand names on the Baby Milk Action website. This strategy considers the likely difficulty in identifying all companies offering products other than CMF which, nevertheless, may be involved in the CMF sector, for instance, through their stakes in other companies manufacturing CMF.
The study will commence after protocol publication. The process of identifying relevant HCPAs will take approximately 1 month from the date of the Gale Directory Library search, with data extraction and analysis expected to be completed in 2024. The exact publication schedule will be determined by the journal editors and reviewers.
Search strategy and screening
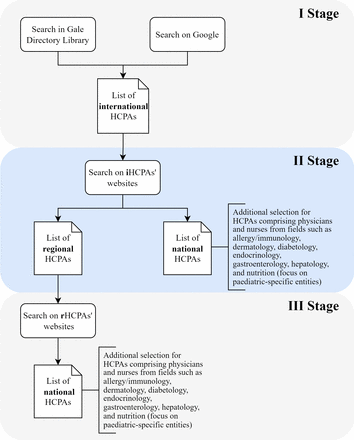
Because international HCPAs often function as umbrella organisations, encompassing regional or national associations, the first stage of our strategy will be to identify these international HCPAs (figure 1). For this purpose, we will use the Gale Directory Library, a comprehensive collection of directories and listings of organisations. To ensure the reproducibility and transparency of our procedure, we will use predefined search criteria and keywords (table 1). To make the list of international HCPAs more exhaustive, we will search Google using the same keywords, and screen the first 20 pages returned for each profession. The browsing cache will be cleared before each search session to ensure that previous searches would not influence the results. We will select international HCPAs with the broadest scope, bringing together other international, regional or national associations, from at least two geographical regions according to the UN statistics division: Africa, Americas, Asia, Europe, Oceania. At this stage, to capture all relevant associations, we will not limit our search strategy solely to those labelled as paediatric. This is because of the variability in medical specialisation systems and regulations across different countries. This approach ensures that we comprehensively account for the diverse global landscape of HCPAs related to our study, before narrowing our focus to associations specific to paediatrics. Two independent analysts will screen the retrieved records, selecting associations based on the inclusion and exclusion criteria detailed in table 2.
Flow diagram showing the three-stage systematic strategy for identifying and selecting healthcare professional associations (HCPAs): from international to regional and national HCPAs with an additional focus on paediatric-specific specialties.
Search strategy with the keywords used for the Gale Directory Library and the Google search engine
Inclusion and exclusion criteria for healthcare professional associations
In the second stage, we will review the official websites of these international HCPAs and collect lists of member associations with a regional (in accordance with the UN division) or national scope. At this stage, for associations comprising physicians and nurses from fields such as allergy/immunology, dermatology, diabetology, endocrinology, gastroenterology, hepatology and nutrition, we will introduce an additional criterion to refine the selection to associations pertaining to paediatric populations. We will differentiate based on whether an international association assembles national or regional associations. If an international organisation consists of national associations, only those with a focus on paediatric populations will be selected for further analysis. However, if the international association gathers regional associations, we will include all such associations in the third stage of selection, regardless of their specific focus in order not to miss any relevant HCPAs.
In the third stage, the same process will be repeated for regional HCPAs to identify any publicly available lists of their national member associations. In the case of associations assembling physicians and nurses from the specific fields mentioned above, we will conduct a selection of their national members to identify those associations whose activities focus on paediatric populations.
Additionally, at each stage, we will consider relevant cooperating or affiliated associations if they are mentioned on the websites of the identified HCPAs but not explicitly defined as their members.
Should associations not have publicly available member lists, they will still be appraised for funding by the CMF industry. In summary, we will analyse international, regional and national associations and collect data from all of them treating them as separate entities.
Data extraction
From the identified HCPAs websites and social media accounts, we will record information on the characteristics of the associations (including names, countries of origin, geographic areas, website addresses and links to their social media accounts), any official documents related to COI, sponsorship or funding policies/rules (eg, codes of ethics, association statutes, guidelines), the type and, if available, the amount of funding from the CMF industry (table 3). Data will be collected in a database and timestamped.
Criteria for identifying and coding conflict of interest policies and types of funding from the CMF industry—draft data extraction sheet (to be piloted)
We will look for any logos or mentions of CMF manufacturers in areas such as acknowledgement sections, partners, lists of financial sponsors or commercial partners, advertisements and lists of conference sponsors or exhibitors. The data extraction search will encompass the main and related pages of the websites, and other available materials such as financial statements/declarations from the organisation about funding sources in general (eg, year-end accounts) or for specific events (eg, annual congresses, workshops, study days, training programmes for professionals or the public, research programmes), presentations, event photos, brochures, educational documents, newsletters and journals. We will not review content that necessitates membership or a paid subscription, such as subscription journals. The evidence of funding will be collected based on its purpose, following an established methodology,15 and classified as either ‘sponsorship’ or ‘payment for services’ (table 3). Funding will be considered ‘sponsorship’ if there appears to be no specific services provided to the donor other than acknowledgement. Funding will be considered ‘payment for services’ if the HCPA provides direct benefits to the company, such as through advertisements in a publication or exhibition space at a conference.15 Evidence of funding will be searched until the first hit in a given category listed in table 3.
Our strategy for documenting evidence of CMF industry sponsoring is based on a hierarchical approach to information sources. We will begin by checking the official website of the identified HCPA. If we find relevant evidence there, we will not proceed to other social media platforms, as previous research has shown that searching Facebook accounts, when official websites are available, does not yield any additional information regarding sponsoring.15 However, if the official website does not provide the necessary information, we will continue our search on Facebook. Should we still not find the required data on Facebook, we will then move on to X (formerly Twitter) and as a last resort, Instagram. The sequence of these sources may be adapted to the availability of accounts on various social media platforms held by a given association or their accessibility in specific countries or regions. We will consider regional equivalents of these platforms, if necessary, for example, using an equivalent of Facebook (WeChat in China or VKontakte in Russia) or X (Sina Weibo in China).
Websites in languages other than English will be translated using Google Translate. When collecting timestamped data from websites and social media accounts, we will include information reaching back up to 24 months from the date of the search.
Key web results will be recorded with the Wayback Machine (https://web.archive.org/save), which allows to store a timestamped version of a website for future reference, as well as to access previous versions of the same page stored by other users in the past. All available materials, statements, regulatory and legal documents related to funding on the websites will be saved as timestamped PDF files.
We will implement a formal concordance process as part of our methodology to ensure the reliability and validity of our data collection, as recommended by Cochrane Handbook in Chapter 5.22 This process will involve:
Pilot phase: two independent researchers involved in the pilot phase will identify data according to a predefined data extraction sheet from 10 randomly selected websites of identified associations. This will allow us to refine our methods and criteria for data extraction.
Reliability of data collection and consensus building: the extracted data will then undergo cross-verification. In cases of discrepancy or disagreement between researchers, a structured consensus process will be employed. This may involve discussion and consultation with a third, senior researcher to resolve any differences in interpretation or findings.
Training and calibration: if additional researchers are involved, beyond those who participated in the pilot phase, training will be provided prior to the start of data extraction to familiarise them with the standardised criteria. To further minimise variability in data extraction and interpretation step 2 will also be applied.
The final phase of the study: data extraction will be conducted independently by two researchers. In case of discrepancies in data extraction, a consensus decision will be made involving a third researcher if necessary.
Data analysis
All data will be collected using a predetermined electronic extraction sheet (see table 3 for key data items). We will use narrative summaries and visual displays to synthesise the data. We will conduct descriptive statistical analyses, using frequencies and percentages to represent the proportion of professional associations receiving funding from the CMF industry. Where possible, we will explore differences between groups of HCPAs with and without financial support, using non-parametric tests as appropriate.
We will consider the proportion of HCPAs receiving any kind of financial support from the CMF industry as the primary outcome. To further investigate the nature and extent of this financial support, we will conduct an analysis of the types of funding received by the HCPAs from the CMF industry, as categorised in table 3. Depending on the data availability, we will conduct additional comparative analyses for the primary outcome based on characteristics of the associations for example, size, income level and breastfeeding rate in the country, the Code ratification status, presence of COI/sponsorship policies or the amount of funding as detailed in the Data extraction section.
The secondary outcome will be the presence of a COI/sponsorship/funding policies among the associations.
Analyses will be conducted separately for physicians of each medical specialty, nurses/midwives, dietitians/nutritionists and lactation consultants.
Furthermore, we will replicate and update the previous findings concerning the sponsoring of paediatric associations by the CMF industry. We will aim to carry out a comparative analysis to determine whether there have been any changes since the last data collection in 2017.
Patient and public involvement
No patients were involved in the creation of this protocol.
Ethics and dissemination
The method described in this protocol aims to enable a systematic analysis of the funding relationships between the CMF industry and HCPAs, a significant but underexplored area of COI in maternal and child health. Through systematic data extraction and analysis, we aim to highlight areas where policies and guidelines may need to be introduced or strengthened.
The ethical approval for such a systematic cross-sectional assessment is not required and the method is based solely on publicly available data voluntarily disclosed by HCPAs or documented in open access COI policies or financial reports. This reliance on publicly available information is a potential limitation of our method because such an approach may not capture all forms of sponsorship or financial relationships. In addition, there might be variations in the way different associations disclose their financial relationships, making the comparison challenging.
Whether receiving funding from the CMF industry inherently threatens professional standards and ethics remains a contentious issue, as evidenced by the statements issued by various professional associations.23 24 However, the need for transparency in such relationships is crucial and undisputed. To maintain public trust, any advice and guidelines given by HCPAs must remain evidence-based and free from any potential commercial influence.
Our analyses will provide insights into the current landscape of industry funding relationships in this sector and pave the way for future research, policy discussions and practice guidelines to ensure unbiased, evidence-based care. Results of these analyses will be widely disseminated in peer-reviewed journal articles, at international conferences and among the HCPAs. In our dissemination strategy, we will aim at specific specialty journals to increase the impact of these results on different health professions, which might be especially relevant if significant differences arise in their respective levels of funding. To enhance transparency, replicability and verifiability, all data arising from this project will be made publicly available on publication of specific analyses either as supplemental material or via the Open Science Foundation platform, depending on the journal.
Ethics statements
Patient consent for publication
Acknowledgments
We would like to thank Iryna Furda for her help with a previous version of this protocol.
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
X @KHenkeCiazynska, @QuinnGrundy, @Meta_Res_Centre
Contributors KH-C was involved in the investigation, conceptualisation and writing of the original draft of the protocol. BH conceptualised this protocol, secured funding, led the investigation and research processes, supervised the project and wrote the original draft of the protocol. IF, AF, QG, DM, LB and RJB contributed to conceptualisation and writing through review and editing of the protocol. All authors gave critical feedback on the revised protocol and approved the final version of the manuscript. All authors provided final approval for publication and agreed to be accountable for all aspects of this work. The corresponding author attests all listed authors meet authorship criteria and that no others meeting criteria have been omitted.
Funding This work was supported by the Polish Ministry of Science and Higher Education ('The Excellence Initiative – Research University' programme), grant number 0320/2020/20.
Competing interests None declared.
Patient and public involvement Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.