Article Text
Abstract
Introduction Poststroke spasticity (PSS) affects up to 40% of patients who had a stroke. Botulinum neurotoxin type A (BoNT-A) has been shown to improve spasticity, but the optimal timing of its application remains unclear. While several predictors of upper limb PSS are known, their utility in clinical practice in relation to BoNT-A treatment has yet to be fully elucidated. The COLOSSEO-BoNT study aims to investigate predictors of PSS and the effects of BoNT-A timing on spasticity-related metrics in a real-world setting.
Methods and analysis The recruitment will involve approximately 960 patients who have recently experienced an ischaemic stroke (within 10 days, V0) and will follow them up for 24 months. Parameters will be gathered at specific intervals: (V1) 4, (V2) 8, (V3) 12, (V4) 18 months and (V5) 24 months following enrolment. Patients will be monitored throughout their rehabilitation and outpatient clinic journeys and will be compared based on their BoNT-A treatment status—distinguishing between patients receiving treatment at different timings and those who undergo rehabilitation without treatment. Potential predictors will encompass the Fugl-Meyer assessment, the National Institute of Health Stroke Scale (NIHSS), stroke radiological characteristics, performance status, therapies and access to patient care pathways. Outcomes will evaluate muscle stiffness using the modified Ashworth scale and passive range of motion, along with measures of quality of life, pain, and functionality.
Ethics and dissemination This study underwent review and approval by the Ethics Committee of the Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy. Regardless of the outcome, the findings will be disseminated through publication in peer-reviewed journals and presentations at national and international conferences.
Trial registration number NCT05379413.
- REHABILITATION MEDICINE
- STROKE MEDICINE
- NEUROLOGY
- PAIN MANAGEMENT
- Quality of Life
- Clinical trials
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
STRENGTHS AND LIMITATIONS OF THIS STUDY
Real-world outcomes will offer insights into the impact of botulinum toxin on upper limb poststroke spasticity concerning timing.
The impact of physical therapy and other potential modifiers of upper limb spasticity, including pharmacological therapies and comorbidities, will be assessed.
There is a consistent risk of drop-outs and missing data due to disability-related patient non-adherence to follow-up visits.
Patients with poststroke disabilities other than upper limb spasticity will not be excluded, adding potential sources of variability as a limitation for the study.
Introduction
Poststroke spasticity (PSS) develops following cerebrovascular lesions, with an incidence and onset time that vary and are only partially predicted by aetiology and patient clinical features.1 Up to 40% of patients may develop PSS within 3–6 months after a stroke, with 20% experiencing severe disability as a result.2 Factors such as more severe paresis at baseline, somatosensory deficits, large strokes, small lesions involving the internal capsule or lenticular structures and lower performance status may partially identify patients at higher risk of developing PSS.3–5 Therefore, predicting the onset or individual risk of developing PSS may enable early measures for prevention or intervention.
Stroke has an incidence rate in Europe ranging between 60 and 200 per 100 000 person-years.6 The prevalence among the EU population is increasing due to factors such as ageing. Interventions can reduce morbidity, mortality and disability-adjusted life-years; thus, managing stroke sequelae is crucial in the realm of neurological chronicity and planning models of care.6 In the Italian Lazio region, regulatory agencies estimate approximately 10 000 patients per year with stroke, of which around 80% experience ischaemic strokes, often involving the territory of the middle cerebral artery and potentially affecting the upper limb.7 8 Spasticity develops in about 30%–50% of stroke patients within 6 months.9 Therefore, upper limb PSS was selected as a model for spasticity study, prediction and follow-up due to its epidemiological relevance and complexity compared with other types of spasticity in terms of body distribution and aetiology.
Poststroke management is highly multidisciplinary, aiming to early identify structural and functional impairments, assess the patient limitations in activities of daily living and design a targeted rehabilitative approach delivered across multiple assessments (eg, hospitals, rehab facilities, outpatient clinics, at home) over time towards recovery.10 11 In this diverse environment, predicting and recognising spasticity is a crucial opportunity to identify and treat PSS, which is a potential factor contributing independently to progressive patient disability.12–14
Botulinum neurotoxin type A (BoNT-A) is approved for spasticity treatment, improving passive and, in selected cases, active functioning by limiting aberrant mechanisms of muscle hyperactivity and modulating propriospinal reflexes and proprioception.15 Treatment involves locally injecting diluted BoNT-A formulations to manage focal or segmental spasticity patterns aligned with patient-oriented goals. BoNT-A treatment has theoretically the potential to alter the time course of PSS.16 Timely execution of BoNT-A therapies may have a significant impact on PSS, in terms of improvement of passive and active functions, and quality of life (QoL).17 However, BoNT-A injections are often administered late after the onset of PSS, and despite available knowledge suggesting higher-risk subjects, injections are frequently performed without proper timing or in advanced cases with low expectations of improving active functions or QoL. Despite BoNT-A having an A level of evidence in the treatment of spasticity, the access of PSS patients to therapies is still suboptimal.18 For instance, data from the French National Hospital Discharge Database revealed that 10% of stroke survivors were coded as having PSS, with only 2.3% of them receiving one or more injections of BoNT-A between 2014 and 2020. This percentage further decreased when considering patients who received three or more injections within the 12 months following BoNT-A treatment initiation, aligning with PSS treatment recommendations, occurring approximately once every 3–4 months.18 Similarly, findings from the USA TriNeTx repository (www.trinetx.com; A global federated real-world data and analytics platform for research %7C JAMIA Open %7C Oxford Academic, oup.com) indicated that only 8.7% of patients diagnosed with a stroke and subsequently diagnosed with PSS in 2023 were treated with BoNT-A injections. Our understanding of the real-world poststroke patient path and the clinical impact of timely botulinum toxin treatment in PSS patients is still limited. Data comparing early versus late BoNT-A treatments are growing,19–22 but a comparison with the real-world prosecution of rehabilitative protocols alone is still absent. The upper limb PSS is a care model worth exploring due to its frequency and related clinical complexities.23 While the patient accessibility to BoNT-A treatments for PSS is acceptable, the possibility of receiving early and timely treatments in a real-world setting is still controversial and dependent on the environment. Prompt treatment may help maintain a lasting effect due to higher but well-tolerated injection doses,24 potentially improving the natural wearing off of BoNT-A that occurs at 12–16 weeks—a major negative factor impacting the QoL of patients treated with botulinum neurotoxins.25 Selecting and delivering BoNT-A treatment for PSS patients is a collaborative effort involving various healthcare professionals along the poststroke patient pathway.26
The COLOSSEO-BoNT (or just COLOSSEO) study protocol aims to investigate how clinical predictors can guide early identification and treatment of upper limb PSS with BoNT-A. Additionally, it aims to determine if such intervention can improve spasticity, functionality, pain, and QoL over time, and the patient care pathway.
Methods
Study design
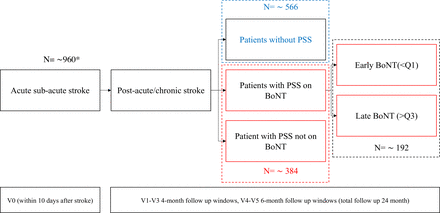
The COLOSSEO study is a multicentre observational prospective cohort study on the development and treatment of upper limb PSS, including the on-label administration of onabotulinum, abobotulinum or incobotulinum neurotoxin type A, following routine clinical practice. Enrolled subjects with acute ischaemic stroke will receive a regular follow-up throughout the real-world poststroke path of care according to the Italian health system (figure 1). Given the real-world setting of this study, randomisation or blinding procedures were not deemed appropriate.
The COLOSSEO-BoNT study flow chart. *Estimated sample size across time points and stratification; Q1, first quartile of time from stroke distribution; Q3, third quartile of time from stroke distribution. BoNT, botulinum neurotoxin type A; PSS, poststroke spasticity.
Study population
This study will observe adult patients with stroke admitted to the stroke units located in the Lazio region, Italy. Stroke units are organised according to a spoke–hub paradigm. This model identifies hub and spoke hospitals with dedicated beds and instrumentation for acute cerebrovascular pathologies. Interaction between the nodes of this system is essential in the acute phase to deliver urgent treatments.27 The COLOSSEO study will take place in the poststroke setting, beginning with the diagnosis and treatment of acute ischaemic stroke involving the upper limb and will follow patients in the acute, postacute and chronic stages of stroke management for up to 24 months. Patients will be recruited based on the inclusion and exclusion criteria listed in table 1.
Inclusion and exclusion criteria of the COLOSSEO study
Screening and recruitment
Participants will be recruited among consecutive admissions to the stroke units. Selected subjects with ischaemic stroke and involvement of the upper limb will be recruited within 10 days of the cerebrovascular event. Patients will be excluded if severe neurological syndromes or ongoing comorbidities during the acute setting will hasten the prosecution of postacute care (table 1). All subjects will be selected as naïve to BoNT-A in their first-ever clinical stroke with involvement of the upper limb. The protocol has been designed and reported according to ‘Strengthening the Reporting of Observational Studies in Epidemiology Statement: guidelines for reporting observational studies’.28
Data collection
All patients will undergo an initial assessment, including a practical set of recognised clinical predictors and will be ecologically followed during their rehabilitation and outpatient clinic management, with a five-visit follow-up scheme (outlined below and figure 2). Throughout their follow-up, patients will be categorised based on whether they received BoNT-A, allowing for a comparison. Those undergoing BoNT-A treatment will be further categorised based on the timing of treatment, distinguishing between early (first quartile) and late (last quartile), enabling a more in-depth comparison. Collected data will serve as possible predictors of spasticity development during the follow-up.
Schematic description of study visits and collected information. (A) Anamnestic information including age, sex, hand dominance, heigh, weigh and BMI, smoking habits, presence of diabetes, atrial fibrillation, hypertension, carotid pathology, chronic obstructive pulmonary disease, chronic kidney injury, seizures, coronary artery disease and related therapies (anticoagulants, antiplatelet agents); (A’) update on comorbid condition and related therapies; (B) Radiological predictors of spasticity including stroke size and location, internal capsule involvement, Fazekas score; (C) Clinical predictors of spasticity including: NIHSS, modified Rankin scale, Barthel index, MMSE, Fugl-Meyer Assessment, baseline modified Ashworth scale; (C’) update on clinical predictors; (D) spasticity outcome measures and related index of functionality and quality of life: modified Ashworth scale, Arm Activity measures (ARMA) scale, Euro-Quality of life 5 Dimension (EQ-5D) scale, pain scale (visual analogue scale, VAS); (E) information on BoNT-A therapy: BoNT-A type, dosage, selected muscles and use of guidance; (F) concomitant therapies including rehabilitation: physical therapies setting, the number of rehabilitation treatments per week, access to robotic rehabilitation, use of spasticity medications. BoNT-A treatments could be recorded at any time during the follow-up as extra visits (EV). Adverse events are collected at any time point and visit. BMI, body mass index; BoNT-A, botulinum neurotoxin type A; MMSE, Mini-Mental State Examination; NIHSS, National Institute of Health Stroke Scale.
Prediction measures
Clinical examinations and the annotation of scores by potential predictors will be conducted at baseline (V0) and during follow-up visits. The NIHSS and the Fugl-Meyer Assessment will be used to evaluate the severity of the poststroke motor and sensory syndrome.29 Other associated neurological signs, such as language impairment and neglect, will also be specifically documented and rated. Muscle stiffness will be assessed using the Modified Ashworth Scale (MAS), along with the analysis of passive range of motion (PROM).26 The Mini-Mental State Examination will gauge the severity of cognitive issues at baseline and during follow-ups while the Barthel Index (BI) will be employed to assess residual functional capacity throughout the study.
Additional data, including the side and the size of the lesion, the occurrence of capsular involvement, and the Fazekas score, will be collected through axial MRI fluid-attenuated inversion recovery at baseline.30 31 Comorbidities and therapies will be documented throughout the study to estimate the role of concurrent medication and past or concomitant comorbidities in predicting the onset of PSS.
Primary outcome and associated measures
To establish the variable or the combination of variables able to predict the onset of upper limb PSS in a real-world setting through the elbow-wrist flexors MAS and PROM.
To evaluate the effect of BoNT-A on improving MAS and the QoL of treated patients versus naturally untreated patients.
Secondary outcome measures
Changes in muscle stiffness and passive mobility scores (MAS and PROM) will be assessed in patients receiving early versus late BoNT-A treatment, stratified based on quartiles (I vs III) of the time distribution between the stroke and the treatment.
Functional scores (ARM-A), QoL (EQ-5D) and pain (visual analogue scale, VAS) will be examined for changes in patients undergoing early versus late treatment.
Changes in muscle stiffness and passive mobility scores (MAS and PROM), as well as functionality (ARM-A), QoL (EQ-5D) and pain (VAS) scores, will be compared between patients with and without BoNT-A treatment.
Parameters will be gathered at specific intervals, namely at 4 months following enrolment (V1), at 8 months (V2), at 12 months (V3), at 18 months (V4) and at 24 months (V5) following the stroke. MAS data will be collected at baseline to determine the prevalence of spasticity in the early poststroke setting. The MAS score at baseline may serve as a predictive factor for spasticity and severe spasticity at follow-up, in line with current literature. The outcomes are categorised into various domains, including physical outcomes (MAS, PROM), functional and QoL outcomes (Euro-Quality of life 5 Dimension (EQ-5D), ArmA, pain VAS),32 educational outcomes (investigator diagnostic confidence level in predicting spasticity), other medical outcome measures (radiological qualitative data, anamnestic and comorbidity findings) and therapeutic outcomes (BoNT-A injection features, treated muscles and dosages; the use of peroral or other injective myorelaxants; rehabilitation intensity at various time points).
BoNT-A injections will be administered by providers/investigators as part of routine clinical practice. Treatment details will be documented during follow-up visits (V0-V5) or as extra visits conducted by the investigators. Adverse events (AEs) and events leading to study discontinuation and/or death will be collected as outlined below.
Treatments and treatment-related outcome measures
BoNT-A injections
BoNT-A injections act through the blockage of the acetylcholine release by alpha motor neurons at the neuromuscular junction and gamma motor neurons at the neuromuscular spindles, favouring muscle relaxation. Moreover, BoNT-A modulates on the release of pain neurotransmitters at a central and peripheral level, improving nociception.15 The treatment is delivered through intramuscular injections, with the aim of targeting pathological focal or segmental stiffness.26 In COLOSSEO, BoNT-A injections will be administered following standardised procedures, in accordance with healthcare professional information leaflets, international guidelines and local regulations, by trained physicians working in BoNT-A clinics within Neurological and Physical Medicine and Rehabilitation departments, as well as outpatient services in the Lazio region, Italy. The use of injection guidance techniques (eg, ultrasounds, electromyography, electrical stimulation) versus a palpation/landmark-based approach will not be mandatory.33–35 All commercially available BoNT-A formulations, such as onabotulinumtoxin, incobotulinumtoxin and abobotulinumtoxin, will be included, reflecting common clinical practice. Details such as muscle and toxin selection, dosage per muscle and the type of guidance employed will be meticulously recorded in case report forms.
Rehabilitation intensity and number of treatments
The rehabilitation patient care pathway is significantly influenced by the resources allocated to the respective territory within the chronic disease treatment plan of the national health system. This becomes particularly relevant in the postacute phase and outpatient clinics or home rehabilitation services catering to highly disabled patients. In the Italian national health programme, the possibility of undergoing a reimbursed poststroke acute residential rehabilitation programme is determined by diagnostic congruency (based on temporal and clinical correlation criteria) and the complexity established by the diagnosis (ie, stroke) and the patient’s performance status (eg, BI). The latter allows the patient to be admitted to a residential intensive rehabilitation department for cases of high complexity or low complexity, or to a residential extensive rehabilitation department for cases of low complexity. Cases of low complexity and severity could also be addressed in the postacute setting through semiresidential services such as day hospitals and outpatient facilities. For comprehensive information, please refer to https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=85585&parte=1&serie=null). The number of sessions per week will be documented along with other relevant details (eg, rehab facilities, access to robotic rehabilitation).
Medication for spasticity
Previous studies identify that the use of medication for spasticity include skeletal muscle relaxants (eg, dantrolene sodium, baclofen) benzodiazepines (eg, diazepam) and alpha2-adrenergic agonists (eg, clonidine, tizanidine) as associated with severe spasticity.36 Medications, including antiepileptics, will be collected and adopted as a therapeutic outcome measure.
Adherence and compliance to cures
Patients entering the poststroke care rehabilitation path will be observed throughout their journey across the various available levels of care (inpatient departments, day services, outpatient clinics and at-home assistance). The time spent on rehabilitation activities will be measured as the number of sessions per week at any time point in any facility.
Given the observational nature of the present protocol, it will not influence the prescription of physical therapy. There will be no ‘a priori’ restriction on allowed patient activity. Patients will be permitted to engage in active and PROM exercises, stretching and any other exercises after BoNT-A treatment. Poststroke training or rehabilitation on the spastic or unaffected limb will be monitored throughout visits. Specifically, the rehabilitation dosage (type and frequency of sessions per week) will be measured as a covariate during the 24-month follow-up, according to clinical routine, to assess its potential influence on spasticity compared with BoNT-A treatment.
Patient and public involvement
The COLOSSEO study was designed with a patient partnership strategy and as an answer to clinical research to the patient’s demand for a solution to the poststroke care fragmentation (https://www.cittadinanzattiva.it/notizie/11898-presentata-raccomandazione-civica-su-spasticita-post-ictus.html). Hence, investigation centres cover almost all the Lazio region of Italy and included almost all the stroke units and the physical medicine and rehabilitation (PMR) departments. Study progress will be presented at meetings hosted in the local health system facilities, between investigators and providers. Once trial results are published, participants will be informed through a study newsletter designed for a non-specialist audience. Investigators will receive regular updates throughout the study via monthly newsletters, providing information on the recruitment process and including aggregated and anonymised data. The COLOSSEO study group has established an educational and clinical network among stroke unit physicians, PMR specialists and BoNT-A neurologists, aiming to enhance the care of patients with PSS.
Study setting and timeline
This study will be conducted in neurology departments/stroke units for recruitments and outpatient clinics for follow-up visits, in PMR departments for recruitments or outpatient clinics for follow-up visits, and in the BoNT-A clinics for follow-up visits and injection extra visits. Recruitment started in July 2022, and all patients are expected to be included before July 2024. The study duration includes a 2-year follow-up, and all examinations will be completed by the end of 2026.
Statistical analysis plan
Analysis will be performed based on the expected incidence of spasticity9 and on the expected rate of treatment with BoNT-A per protocol basis.18 At study closure, variables will be investigated for their distribution (ie, whether parametric or not) through the Shapiro-Wilks test. According to the distribution, continuous data will be presented as mean (SD) or median (CIs), while number (frequencies) will be adopted in the case of categorical variables. Between-group comparisons of clinical scores from baseline to follow-up will be analysed using the difference between means (t-test or Wilcoxon signed rank), and analysis of variance for repeated measures and linear mixed models to test the effect of treatment, with post hoc corrections for multiple comparisons. Categorical variables will be tested through the χ2 test. The association between measures will be investigated through correlation tests (Pearson’s or Spearman’s method), and variables significantly associated will be further investigated through regression analysis, if the assumption for regressions will be fulfilled. Time-to-event analysis will be performed when appropriate using Cox regression models. Group comparisons for the timing of BoNT-A injections will be conducted between the first (I) and the third (III) quartile of the distribution of time at injections. A p value of 0.05 will be adopted for statistical significance. Missing data will be analytically reported and managed according to the multiple imputation method.37
Safety of the poststroke patient path and of BoNT-A treatment and reporting of AEs
Given the observational nature of the study, there are no specific risks to participating in the COLOSSEO study. Patients will be all naturally exposed to the increased morbidity of the stroke and of the poststroke phase38 and adverse events (AEs). Moreover, the subgroup of subjects undergoing BoNT-A treatment will be specifically exposed to the possibility of reporting AEs of receiving injections (eg, pain, bleeding) and of the action of BoNT-A (eg, pain, weakness and swelling).39 However, given that treatments will be designed by experienced injectors according to the patient’s need (no fixed protocols or doses per treatment), we assume that the rate of BoNT-related AEs will be lower than in published interventional studies. AEs will be monitored by the research group throughout the 24-week intervention period (follow-up visits and extra-isits according to the patient path of care). Participants in both the intervention and control groups will also receive a contact number to study staff in the occurrence of AEs. All details of AEs which occur will be documented according to patient safety procedures by the investigator.
The subsequent definitions will be employed in documenting AEs. (1) AE: any unfavourable medical incident in a patient or subject participating in a clinical study; (2) Serious AE (SAE): any unexpected and unfavourable medical incident or effect that results in death, poses a life-threatening situation-pertaining to an occurrence where the subject was at risk of death during the event (ie, it excludes events that might have hypothetically caused death if more severe), requires hospitalisation or extends the ongoing hospitalisation of inpatients, results in lasting or significant disability or incapacity, involves a congenital anomaly or birth defect. Medical judgement will be applied to determine whether an AE is serious in other scenarios; (3) Significant AEs: not immediately life-threatening or fatal, and not resulting in hospitalisation, but which may endanger the subject or necessitate intervention to prevent outcomes listed in the definition above, will also be regarded as serious. All AEs will be reported, and the reporting procedures outlined below will be followed depending on the nature of the event. All non-serious AEs, whether anticipated or not, will be documented. All SAEs will be collected and recorded, whether they are ‘related’ meaning they resulted from the administration of any research procedures, or ‘unexpected’, denoting an event that is not an anticipated occurrence.
Discussion
The significant global prevalence of stroke, particularly in the European Union, underscores the potential to improve morbidity and mortality rates through early interventions and preventive measures during the acute phases. This emphasis brings attention to the evolving challenges posed by cerebrovascular pathologies in the chronic stroke phase.6 40
Mechanisms behind PSS involve progressive short-term and long-term changes in endogenous brain plasticity initiated by brain lesions, inflammation and scarring, making it a dynamic phenomenon with variability in its onset time after a brain lesion.41 42 Moreover, PSS develops on pre-existing neurological impairments, such as limb paresis and can independently contribute to a growing physical disability burden.43 Indeed, spasticity, directly or indirectly, causes debility through mobility impairment, altered postures, deformities, risk of pressure ulcers and infections, disturbed sleep and fatigue, and pain—the latter being a crucial element to identify, treat and monitor in conjunction with the motor impairment due to its significant impact on QoL and potential treatability.44 The presence of PSS adds to the physical disability, diminishes the QoL, engages caregivers and deviates from the intended rehabilitative pathway.12 The timely recognition of spasticity and the early administration of optimal medical therapies become imperative for physicians managing survivors of cerebrovascular diseases, especially during the acute phases, when the fate of PSS development is potentially determinable.2 9 29 36
This study focused specifically on upper limb spasticity resulting from ischaemic stroke. This decision was made to avoid introducing additional variables such as haemorrhagic strokes, traumatic brain injuries, multiple sclerosis and spinal lesions. By concentrating on upper limb PSS, we aimed to investigate a relevant model of care due to its significant epidemiological impact and the complexity associated with this specific condition. The multicentric COLOSSEO study has gathered 15 participant institutions within Rome and the Lazio region, involving all the major local health agencies of Rome and Lazio, instilling confidence in recruiting and following up a large sample of patients. This aligns with the natural diagnostic and therapeutic care pathway for upper limb PSS. After the acute phase, patients with chronic stroke sequelae, including weakness, unsteadiness or early spasticity, are directed to inpatient rehabilitation departments for residential or semiresidential physical therapy and medical care in the postacute phase. Subsequent clinical follow-ups, typically occurring within 6–12 months after a stroke, are often sought in outpatient clinics or through at-home services. A comprehensive 24-month follow-up per patient enables the research group to depict the entire PSS patient pathway, offering sufficient insights for patients treated with BoNT-A even at later phases. It also allows an analysis of the health system’s capability to provide timely treatment within the therapeutic bounds of BoNT-A.15 25 The study will also explore functional and QoL outcome measures, providing valuable real-world insights, despite the necessity for prospective double-blind clinical trials to establish clear class I evidence on the effectiveness of BoNT-A compared with rehabilitation and varying injection times in improving functionality and QoL for PSS patients.
Trials on early treatment with BoNT-A have left several unanswered questions that require addressing before this approach can be widely adopted in patients with chronic stroke and PSS. Furthermore, additional data will support the integration of available PSS prediction strategies as routine practices in stroke units and acute medicine departments.14 Early patient stratification would assist clinicians in making informed decisions about proper patient selection and addressing specific goals for each patient, considering pharmacoeconomic factors.45 Additionally, more data on pain would help further elucidate the impact of BoNT-A on this key parameter of QoL in the long term.46 The ongoing observational prospective trial, COLOSSEO, is designed to provide crucial information about the real-world PSS patient pathway and the impact of botulinum toxin injection therapy on chronic stroke patients in Italy, offering insights into the European Union health system.
The trial’s design encompasses several clinical endpoints, with the elbow-wrist flexors MAS and PROM chosen as primary measures. Exploratory items include spasticity and functional assessments such as Arm-A, QoL and pain evaluations. Notably, there is currently no clear consensus or definition regarding the appropriate timing for botulinum toxin intervention to treat spasticity, with a potential cut-off of 3 months from onset considered for defining early treatment. Finally, considering the uneven distribution of botulinum toxin provisions in Italy, particularly in the Lazio region, analysing this specific territory will provide insights into the system and help avoid biases introduced by real-world studies of larger territories.47
This study has a real-world observational design, which presents a primary limitation: a significant risk of dropouts. This risk may naturally increase over time due to the anticipated progression of the patient disability. However, the meticulous collection of early termination and end-of-study information will allow us to analyse these events as potential additional outcomes. Other potential sources of post-stroke acquired disability (e.g., lower limb spasticity, language disturbances and neglect) might introduce additional sources of variability. Their use as covariates will be considered for post hoc analyses. To mitigate interrater variability of clinical scales, focused trainings and investigator meetings will be conducted.
Ethics and dissemination
This study underwent a thorough review and received approval from the Ethical Committee of Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy which serves as the study promoter. Additionally, approval was obtained from other participating centres. The study and data protection processes will strictly adhere to the European General Data Protection Regulation (GDPR EU) 2016/679 and the Italian Act on Data Protection (law n. 101/2018), along with directives outlined in the official gazette n.205, dated 4 September 2018.
All participants have provided written informed consent and maintain the freedom to withdraw from the study at any time without the need to provide a reason, and this decision will not impact their ongoing treatment. In the event of any AEs, whether related or unrelated to the trial, prompt treatment will be administered, and comprehensive records will be maintained for all participants. AEs will be duly reported to the ethical committee and relevant authorities in accordance with Italian law.
The commitment is made to disseminate and publish the study results, irrespective of whether they are positive, negative or inconclusive. The findings will be shared in interdisciplinary journals and will be presented at both international and national conferences. Furthermore, complete results from the clinical trial will be made publicly accessible on ClinicalTrials.gov under the identifier NCT05379413.
Ethics statements
Patient consent for publication
References
Footnotes
Collaborators The COLOSSEO study group: Alessandro Magliozzi, Stefano Toro, Gaia Anzini (Unit of Neurology, Neurophysiology, Neurobiology and Psychiatry, Department of Medicine, University Campus Bio-Medico of Rome, Rome, Italy; Fondazione Policlinico Universitario Campus Bio-Medico, Rome, Italy); Claudia Celletti, Marco Andrighetti, Paolo Amisano, Marco Falletti (Sapienza University of Rome, 00185 Rome, Italy); Marina Diomedi (University of Rome "Tor Vergata", Via Montpellier 1, 00133, Rome, Italy); Pierandrea Rizzo (Fondazione Policlinico Universitario A. Gemelli IRCCS - UOC Neurologia, Rome, Italy); Luigi Polidori, Grazia Libutti (Unit of Neurology, San Filippo Neri Hospital, ASL RM1, Rome, Italy); Marilena Mangiardi, Francesca Romana Pezzella, Silvia La Cesa, Claudio Gasperini (San Camillo-Forlanini Hospital, Rome, Italy); Marina Cao (Sant’Andrea University Hospital, Sapienza University of Rome, Via Di Grottarossa, 1035, 00189, Rome, Italy); Francesco Asci (Unit of Neurology, San Camillo de Lellis Hospital, Rieti, Italy); Serena Capobianco (CTO hospital, ASL RM2, Rome, Italy); Luisella D’Angeli (Physical Medicine and Rehabilitation unit, Poggio Mirteto, ASL Rieti); Caterina Galluccio (IRCCS Fondazione Don Carlo Gnocchi, Florence); Deepak Gupta (Department of Neurological Sciences, Larner College of Medicine, University of Vermont, Burlington, VT, USA).
Contributors MM, AS, DT and MCA study conception and design; MM wrote the first draft, MM, AS, DT and MCA, MGP, EC, GF, RB, VR, AA, MB, SA, DCrupi, MG, AM, EM, MCM, LC, FB, LM, DS, IA, EI, DCentonze, FP and VDL reviewed and critique the manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient and public involvement Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review Not commissioned; externally peer reviewed.